注:本文为 “原子轨道 · 电子构型” 相关概述和讨论合辑。
英文引文,机翻未校。
略作重排,未整理去重。
如有内容异常,请看原文。
The Origin of the s, p, d, f Orbital Labels
s、p、d、f 轨道标记的起源
William B. Jensen
威廉·B·延森
Department of Chemistry, University of Cincinnati Cincinnati, OH 45221-0172
辛辛那提大学化学系,俄亥俄州辛辛那提市,邮编 45221-0172
Question
问题
Why are the letters s, p, d and f used to label electronic subshells?
为什么使用字母 s、p、d、f 来标记电子亚层?
Ian D. Rae
伊恩·D·雷
16 Bates Drive
贝茨街 16 号
Williamstown, Victoria 3016
威廉斯敦,维多利亚州,邮编 3016
AUSTRALIA
澳大利亚
Answer
回答
Writing in 1937, the British physicist, A. C. Candler, divided the history of spectroscopy into four eras, which he called the acoustics period, the series period, the old quantum period, and the newer quantum mechanical period (1, 2). “The first period,” Candler observed, “began with the earliest measurements of wave lengths and continued in the work of Boltzmann, Liveing and Dewar until 1881 … During this period any theories put forward were based on analogies with the harmonic ratios of sound.” It is in this period that the story of s, p, d, and f begins and it does so with the work of the last two scientists mentioned by Candler - the British chemists, George Liveing and Sir James Dewar, who published roughly a dozen papers between 1872 and 1880 dealing with the line spectra of the alkali metals (3). In describing these spectra, Liveing and Dewar took to qualitatively characterizing the various lines in terms of both their intensity and definition as being either principle, sharp, or diffuse, and further noted that lines of a given type appeared in groups or series.
1937 年,英国物理学家 A·C·坎德勒(A. C. Candler)将光谱学的历史划分为四个时期,他将其分别命名为声学时期、谱线系时期、旧量子时期和新量子力学时期(1, 2)。坎德勒指出:“第一个时期始于最早的波长测量,随后在玻尔兹曼(Boltzmann)、莱文(Liveing)和杜瓦(Dewar)的研究中延续至 1881 年……在此期间,所有提出的理论都是基于与声音谐波比的类比。” s、p、d、f 的起源故事便始于这一时期,其源头是坎德勒提到的后两位科学家——英国化学家乔治·莱文(George Liveing)和詹姆斯·杜瓦爵士(Sir James Dewar)的研究。1872 年至 1880 年间,他们发表了约 12 篇关于碱金属线光谱的论文(3)。在描述这些光谱时,莱文和杜瓦开始根据强度和清晰度,将不同谱线定性地分为“主线(principle)”、“锐线(sharp)”和“漫线(diffuse)”三类,并进一步指出,特定类型的谱线会以组或系的形式出现。
Stimulated by Johann Balmer’s discovery in 1885 of an empirical formula interrelating the four principle spectral lines of hydrogen, Candler’s second or “series” period was, as suggested by its name, characterized by attempts to extend Balmer’s approach to other elements. In the case of the alkali metals, this work was done largely by the German team of Heinrich Kayser and Carl Runge (4), and also independently by the Swedish spectroscopist Johannes Rydberg (5). Their work demonstrated that many of the lines in the spectra of the alkali metals could be mathematically modeled as the sum of three independent series, which Rydberg, following the earlier nomenclature of Liveing and Dewar, named the principle, sharp and diffuse series. In 1907 yet a fourth series of lines was discovered in the spectra of the alkali metals by Arno Bergmann and named the fundamental series (6).
1885 年,约翰·巴尔末(Johann Balmer)发现了一个经验公式,该公式可将氢的四条主线光谱线关联起来。受此启发,坎德勒划分的第二个时期(即“谱线系时期”)正如其名称所示,核心特征是尝试将巴尔末的方法推广到其他元素上。在碱金属的研究中,这项工作主要由德国团队海因里希·凯泽(Heinrich Kayser)和卡尔·龙格(Carl Runge)完成(4),瑞典光谱学家约翰内斯·里德伯(Johannes Rydberg)也独立开展了相关研究(5)。他们的研究表明,碱金属光谱中的许多谱线可以通过数学建模表示为三个独立谱线系的叠加;里德伯沿用了莱文和杜瓦此前提出的命名法,将这三个谱线系分别命名为“主线系(principle series)”、“锐线系(sharp series)”和“漫线系(diffuse series)”。1907 年,阿诺·伯格曼(Arno Bergmann)在碱金属光谱中又发现了第四个谱线系,并将其命名为“基线系(fundamental series)”(6)。

Figure 1. Friedrich Hermann Hund (1896-1997).
图 1. 弗里德里希·赫尔曼·洪德(Friedrich Hermann Hund,1896-1997)。
As is well known, Chandler’s third period was characterized by attempts, starting with Bohr’s famous paper of 1913, to provide a physical model of the atom consistent with the empirical series formulas found earlier. Extending this model from hydrogen to other elements led to the introduction of a variety of more complex quantization schemes, none of which proved wholly satisfactory until the work of Stoner, Main Smith, and Pauli and the introduction of the newer quantum mechanics in the early 1920s (Candler’s fourth period). The history of this eventual resolution is far too complex to deal with in the space available. However, one of its most important consequences was the establishment of our modern electronic atomic configurations and an understanding of their relationship to the periodic table.
众所周知,坎德勒划分的第三个时期(旧量子时期)以构建原子物理模型为核心——从 1913 年玻尔(Bohr)发表那篇著名论文开始,科学家们尝试建立与早期发现的经验谱线系公式相符的原子模型。将这一模型从氢原子推广到其他元素的过程中,人们提出了多种更复杂的量子化方案,但这些方案均未完全令人满意。直到 20 世纪 20 年代初,斯通纳(Stoner)、梅因·史密斯(Main Smith)和泡利(Pauli)的研究取得突破,新量子力学得以确立(即坎德勒划分的第四个时期),这一问题才最终解决。这一最终解决方案的历史过程极为复杂,难以在有限篇幅内详述,但它最重要的成果之一便是确立了现代电子原子构型,并让人们理解了原子构型与元素周期表之间的关系。
J. Chem. Educ., 2007, 84, 757-758
《化学教育杂志》,2007 年,第 84 卷,第 757-758 页
WILLIAM B. JENSEN
威廉·B·延森
This breakthrough is usually attributed to a 1922 monograph by Bohr, but close inspection of Bohr’s configurations shows that his subshell assignments are incorrect (7). In actual fact, our current configurations first appeared in Max Born’s 1925 monograph, Vorlesungen über Atommechanik, though in his introduction Born indicated that both the configuration table and the discussion of its relationship to the periodic table were actually the work of “my assistant Dr. Friedrich Hund” (8). Two years later Hund expanded this work into a monograph of his own entitled Linienspektren und periodisches System der Elemente (9).
这一突破通常被认为源自玻尔 1922 年出版的专著,但仔细研究玻尔提出的原子构型会发现,他对亚层的分配是错误的(7)。事实上,我们目前所用的原子构型最早出现在马克斯·玻恩(Max Born)1925 年的专著《Vorlesungen über Atommechanik》(《原子力学讲义》)中。不过玻恩在引言中指出,书中的构型表以及关于构型与周期表关系的讨论,实际出自“我的助手弗里德里希·洪德博士(Dr. Friedrich Hund)”之手(8)。两年后,洪德将这部分研究拓展,独立出版了专著《Linienspektren und periodisches System der Elemente》(《线光谱与元素周期系》)(9)。
In the version of the configuration table which had appeared in Born’s monograph, Hund (figure 1) had followed Bohr’s practice of labelling the various shells and subshells in terms of their corresponding numerical quantum numbers as
3
1
3_{1}
31,
3
2
3_{2}
32 etc. In his own monograph, however, he replaced the secondary quantum number with the series notations (s, p, d, and f) used by Sommerfeld and others as abbreviations for the characteristic series constant,
μ
\mu
μ, which had appeared in Rydberg’s original empirical equation for the sharp, principle, diffuse and fundamental line series found the spectra of the alkali metals, and instead wrote 3s, 3p, 3d etc. (10). Beginning in the 1930s both Hund’s corrected configurations and his s, p, d, f notation began to slowly leak into the chemical literature, where they have reigned supreme ever since (11).
在玻恩专著中的构型表版本里,洪德(图 1)沿用了玻尔的标记方式:根据壳层和亚层对应的数值量子数,将其标记为
3
1
3_{1}
31、
3
2
3_{2}
32 等。但在他自己的专著中,洪德用索末菲(Sommerfeld)等人使用的谱线系符号(s、p、d、f)取代了角量子数(secondary quantum number)。这些符号是“特征谱线系常数
μ
\mu
μ”的缩写,而
μ
\mu
μ 最早出现在里德伯针对碱金属光谱中锐线系、主线系、漫线系和基线系提出的经验方程中。由此,洪德将之前的标记改为 3s、3p、3d 等形式(10)。从 20 世纪 30 年代开始,洪德修正后的原子构型及其提出的 s、p、d、f 标记法逐渐出现在化学文献中,此后一直被广泛沿用(11)。
Literature Cited
参考文献
-
A. C. Candler, Atomic Spectra and the Vector Model, Vol. 1, Cambridge University Press: Cambridge, 1937, pp. 1-2.
A·C·坎德勒,《原子光谱与矢量模型》,第 1 卷,剑桥大学出版社:剑桥,1937 年,第 1-2 页。 -
For a detailed history of Candler’s first two periods, see W. McGucken, Nineteenth-Century Spectroscopy: Development of the Understanding of Spectra 1802-1897, Johns Hopkins Press: Baltimore, MD, 1969.
关于坎德勒划分的前两个时期的详细历史,参见 W·麦古肯(W. McGucken),《19 世纪光谱学:1802-1897 年光谱认知的发展》,约翰·霍普金斯大学出版社:马里兰州巴尔的摩,1969 年。 -
These mostly appeared in the Proceedings of the Royal Society and are conveniently collected together in G. Liveing, J. Dewar, Collected Papers on Spectroscopy, Cambridge University Press: Cambridge, 1915.
这些论文大多发表于《英国皇家学会会报》,后被集中收录于 G·莱文、J·杜瓦合著的《光谱学论文集》,剑桥大学出版社:剑桥,1915 年。 -
H. Kayser, C. Runge, “Über die Spectren der Alkalien,” Ann Physik, 1890, 41, 302-320.
H·凯泽、C·龙格,《论碱金属光谱》,《物理学年鉴》,1890 年,第 41 卷,第 302-320 页。 -
J. R. Rydberg, “Sur la constitution des spectres linéaires des éléments chimiques,” Comptes rendus, 1890, 110, 394-397.
J·R·里德伯,《论化学元素的线光谱结构》,《法国科学院院报》,1890 年,第 110 卷,第 394-397 页。 -
A. Bergmann, Beiträge zur Kenntniss der ultrarothen Emissionsspectren der Alcalien, Doctoral Dissertation, Jena, 1907.
A·伯格曼,《碱金属红外发射光谱研究》,博士论文,耶拿,1907 年。 -
N. Bohr, The Theory of Spectra and Atomic Constitution, Cambridge University Press, Cambridge, 1922, p. 133. A more complete, but equally incorrect, table appears opposite page 129 in the second edition of 1924.
N·玻尔,《光谱理论与原子构造》,剑桥大学出版社,剑桥,1922 年,第 133 页。1924 年第二版中,第 129 页对面附有一个更完整但同样存在错误的(原子构型)表。 -
M. Born, Vorlesungen über Atommechanik, Vol. 1, Springer: Berlin, 1925, table opposite page 226.
M·玻恩,《原子力学讲义》,第 1 卷,施普林格出版社:柏林,1925 年,第 226 页对面的表格。 -
F. Hund, Linienspektren und periodisches System der Elemente, Springer: Berlin, 1927, table opposite page 55.
F·洪德,《线光谱与元素周期系》,施普林格出版社:柏林,1927 年,第 55 页对面的表格。 -
A. Sommerfeld, Atomic Structure and Spectral Lines, Dutton: New York, NY, 1924, p. 376 (First German edition 1919). Interestingly Sommerfeld used b for Bergmann rather than f for fundamental as his abbreviation for the fourth series.
A·索末菲,《原子结构与光谱线》,达顿出版社:纽约州纽约市,1924 年,第 376 页(德文第一版出版于 1919 年)。有趣的是,索末菲用“b”(对应“伯格曼”的首字母)作为第四个谱线系的缩写,而非“f”(对应“基线系”的首字母)。 -
In the case of introductory textbooks this leakage was often slow. Thus both the fourth (1933) and fifth (1937) editions of James Partington’s, A Textbook of Inorganic Chemistry, continued to use the labeling scheme found in Born and Bohr. Only in the sixth edition of 1950 did Partington finally adopt the s, p, d, f labels of Hund.
在入门级教材中,这种(标记法的)渗透往往较为缓慢。例如,詹姆斯·帕廷顿(James Partington)所著的《无机化学教材》第四版(1933 年)和第五版(1937 年)仍沿用玻恩和玻尔提出的标记方案,直到 1950 年的第六版,帕廷顿才最终采用洪德提出的 s、p、d、f 标记法。
Do you have a question about the historical origins of a symbol, name, concept or experimental procedure used in your teaching? Address them to Dr. William B. Jensen, Oesper Collections in the History of Chemistry, Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172 or e-mail them to jensenwb@ucmail.uc.edu
若您对教学中使用的符号、名称、概念或实验方法的历史起源有疑问,可致函威廉·B·延森博士(地址:辛辛那提大学化学系,化学史奥斯珀收藏馆,俄亥俄州辛辛那提市,邮编 45221-0172),或发送电子邮件至 jensenwb@ucmail.uc.edu。
2009 Update
2009 年更新
Since writing the column, I have discovered that one of the first advanced monographs to employ Hund’s s, p, d, f orbital notation was the 1930 monograph on the periodic table by the German chemists, Eugen Rabinowitsch and Eric Thilo:
撰写本专栏后,我发现最早采用洪德 s、p、d、f 轨道标记法的高级专著之一,是德国化学家欧根·拉比诺维奇(Eugen Rabinowitsch)和埃里克·蒂洛(Eric Thilo)于 1930 年出版的关于周期表的专著:
E. Rabinowitsch, E. Thilo, Periodisches System: Geschichte und Theorie, Enke: Stuttgart, 1930, pp. 244-245.
E·拉比诺维奇、E·蒂洛,《周期系:历史与理论》,恩克出版社:斯图加特,1930 年,第 244-245 页。
2007, 84, 757-758
2007 年,第 84 卷,第 757-758 页
Definition of Sublevel
亚层的定义
What is a Sublevel?
什么是亚层?
A sublevel is an energy level defined by quantum theory.In chemistry,sublevels refer to energies associated with electrons.In physics,sublevels may also refer to energies associated with the nucleus.
亚层是由量子理论定义的能量级。在化学中,亚层指的是与电子相关的能量。在物理学中,亚层也可能指的是与原子核相关的能量。
Niels Bohr’s earliest quantum theory said that electrons occupy spherical shells centered on the atomic nucleus,such as the two electron shells shown for lithium below:
尼尔斯·玻尔最早的量子理论认为,电子占据以原子核为中心的球形壳层,如下图所示的锂的两个电子壳层:
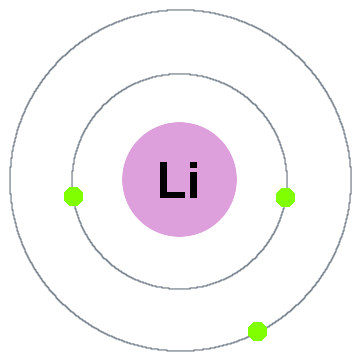
In this old shell model moving outward:
在这个旧的壳层模型中,向外移动:
- Shell 1 can hold up to 2 electrons,
第 1 壳层最多可容纳 2 个电子, - Shell 2 can hold up to 8 electrons,
第 2 壳层最多可容纳 8 个电子, - Shell 3 can hold up to 18 electrons,
第 3 壳层最多可容纳 18 个电子, - Shell 4 can hold up to 32 electrons,
第 4 壳层最多可容纳 32 个电子, - Shell 5 can hold up to 50 electrons,…
第 5 壳层最多可容纳 50 个电子……
Each shell is actually an energy level.The higher the shell,the higher the energy of its electron(s).All the electrons sharing a shell are degenerate,meaning they have the same amount of energy.
每个壳层实际上是一个能量级。壳层越高,其电子的能量就越高。所有共享一个壳层的电子都是简并的,即它们具有相同的能量。
Splitting the Shells into Subshells
将壳层划分为亚壳层
Advances in spectroscopy revealed that shells can actually contain subshells/sublevels.The electrons in shell 3,for example,might have different amounts of energy from one another because they occupy different sublevels.
光谱学的进步揭示了壳层实际上可以包含亚壳层/亚层 。例如,第 3 壳层中的电子可能彼此具有不同的能量,因为它们占据不同的亚层。
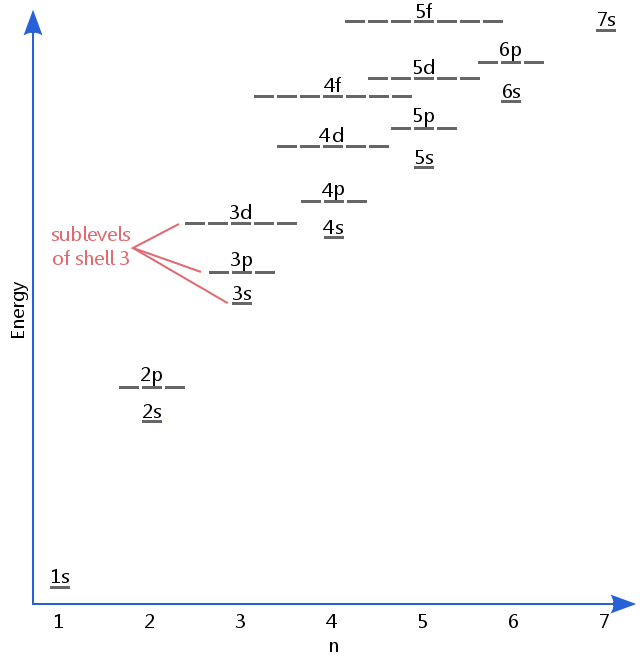
The energy level diagram below shows sublevels to as high as the energy level of the 5f orbitals.Sublevels actually continue to higher energies than this,but 5f is a suitable place to leave an introductory description.
下面的能量级图显示了亚层,直到 5f 轨道的能量级。亚层实际上会延伸到比这更高的能量,但 5f 是一个适合结束入门描述的地方。
Naming the Subshells/Sublevels
命名亚壳层/亚层
Electron sublevels are known by the letters s,p,d,and f.So,for example,electrons in the s sublevel of shell 3 have a different amount of energy from electrons in the p and d levels of shell 3.(This is not the case for hydrogen.All of hydrogen’s sublevels have the same energy,because hydrogen only has one electron.)
电子亚层以字母 s、p、d 和 f 为名。因此,例如,第 3 壳层的 s 亚层中的电子与第 3 壳层的 p 和 d 亚层中的电子具有不同的能量。(对于氢来说并非如此。氢的所有亚层都具有相同的能量,因为氢只有一个电子。)
s,p,d,and f sublevels
s、p、d 和 f 亚层
The sublevel occupied by any electron is determined by the electron’s angular momentum quantum number,l.
任何电子所占据的亚层由其角动量量子数 l 决定。
Angular Momentum Quantum Number and Sublevel Type
角动量量子数与亚层类型
| Angular Momentum Quantum Number,l | Sublevel | Number of electrons sublevel can accommodate |
|---|---|---|
| 0 | s | 2 |
| 1 | p | 6 |
| 2 | d | 10 |
| 3 | f | 14 |
The energy level diagram excerpt below shows the sublevels corresponding to different values of l in the fourth electron shell.
下面的能量级图摘录显示了第四电子壳层中不同 l 值对应的亚层。
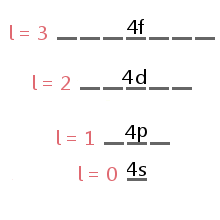
Sublevel orbitals have characteristic shapes that can be used to explain and predict the chemical bonds that atoms can form.These shapes are based on the likelihood of finding an electron at any particular location orbiting the nucleus.
亚层轨道具有特征形状,可用于解释和预测原子可以形成的化学键。这些形状是基于在原子核周围任何特定位置找到电子的可能性。
For example,we can say that an electron in a hydrogen atom’s 1s sublevel will be found 99 percent of the time somewhere in a sphere with a given radius around the nucleus.This is the reason we can draw the s sublevel’s orbitals as a sphere.
例如,我们可以认为,氢原子的 1s 亚层中的电子有 99%的时间会出现在围绕原子核的某个给定半径的球体内。这就是我们可以将 s 亚层的轨道画成球体的原因。
Orbitals for higher sublevels are also drawn on the basis of where an atom’s electrons are most likely to be found.See orbitals for more details.
更高亚层的轨道也是根据原子的电子最有可能被找到的位置来绘制的。更多详情请参见周期表。
An s sublevel’s orbital
s 亚层的轨道
Sublevel Examples
亚层示例
Examples of the sublevels found in various atoms are shown below.The superscript shows the number of electrons in each sublevel.
下面展示了各种原子中存在的亚层示例。上标表示每个亚层中的电子数。
Hydrogen: 1s1
氢 :1s¹
Carbon: 1s2 2s2 2p2
碳 :1s²2s²2p²
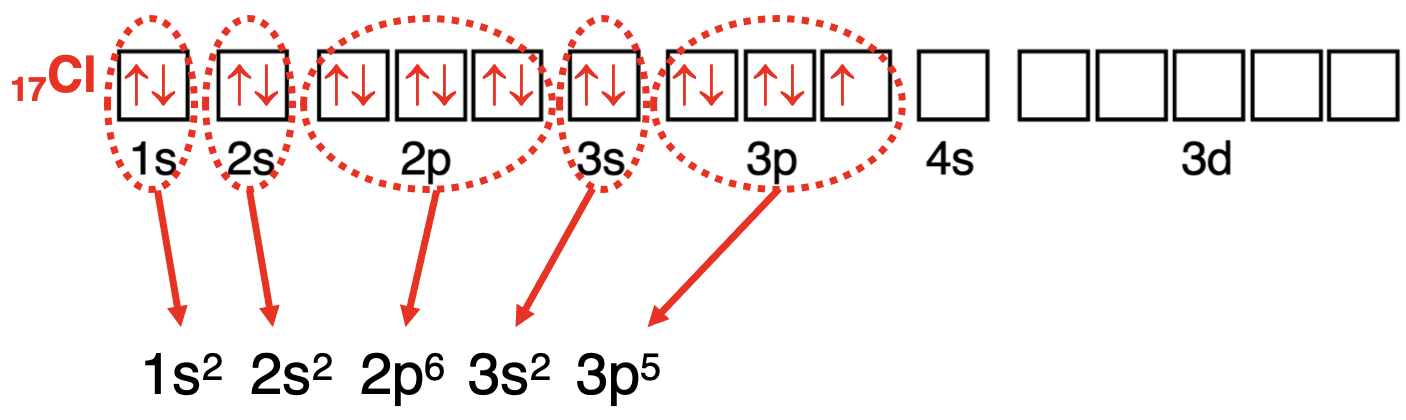
Chlorine: 1s2 2s2 2p6 3s2 3p5
氯 :1s²2s²2p⁶3s²3p⁵
Argon: 1s2 2s2 2p6 3s2 3p6
氩 :1s²2s²2p⁶3s²3p⁶
In general,electrons go into the lowest available energy sublevel.The general order in which sublevels are filled is:
一般来说,电子会进入最低可用能量的亚层。亚层填充的一般顺序是:
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Given this,we can answer questions like: “what are potassium’s electron sublevels?”
鉴于此,我们可以回答诸如“钾的电子亚层是什么?”之类的问题。
Potassium has 19 electrons,and we know that s orbitals hold a maximum of two electrons and p a maximum of six.Potassium’s electron sublevels will be:
钾有 19 个电子,我们知道 s 轨道最多可容纳两个电子,p 轨道最多可容纳六个电子。钾的电子亚层将是:
1s²2s²2p⁶3s²3p⁶4s¹
For some individual atoms with more than 20 electrons the 3d orbitals may fill before the 4s orbitals.Also,there may be other exceptions to this filling order,as discussed in the comments section for iodine.
对于某些具有超过 20 个电子的单个原子,3d 轨道可能会在 4s 轨道之前被填满。此外,这种填充顺序可能还有其他例外,如在碘的评论部分中所讨论的。
Jessica Bickley says
October 30, 2012 at 5:01 pm
Hey there! I noticed a small error on the site regarding the electron configuration of Iodine. It should read: [ Kr ] 5 s 2 4 d 10 5 p 5 [\text{Kr}] 5s^2 4d^{10} 5p^5 [Kr]5s24d105p5 because the 4d energy level is filled before the 5p level. Hope I helped!
您好!我发现网站上关于碘(Iodine)原子电子构型的内容存在一处小错误。碘的电子构型应表示为: [ Kr ] 5 s 2 4 d 10 5 p 5 [\text{Kr}] 5s^2 4d^{10} 5p^5 [Kr]5s24d105p5,因为 4d 能级会在 5p 能级之前填满。希望我的指出能帮到你们!Doug Stewart says
October 31, 2012 at 1:39 pm
Hi Jessica, thanks for your comment. The configuration we’ve shown is actually correct.
您好,杰西卡,感谢您的留言。我们所展示的(碘的)电子构型实际上是正确的。
The Convention
相关惯例
What I should say to start with is that we’ve followed a convention for electron configurations so that, if there are electrons present in orbitals, lower principal quantum numbers are always shown preceding higher principal quantum numbers in the configuration. This means that it doesn’t matter whether in a real atom the 5s has lower or higher energy than the 4d orbitals. We always write 4d before 5s.
首先需要说明的是,我们在表示电子构型时遵循了一项惯例:当轨道中存在电子时,在电子构型的书写中,主量子数(principal quantum number)较小的轨道始终排在主量子数较大的轨道之前。这意味着,在实际原子中 5s 轨道的能量是否低于或高于 4d 轨道,并不影响书写顺序——我们始终将 4d 写在 5s 之前。
In the case of iodine this convention actually does yield the correct electron configuration – although it may at first seem to disagree with the orbital energy levels shown in textbooks, which show the filling order for orbitals as:
对于碘原子而言,遵循这一惯例恰好能得到正确的电子构型——尽管初看之下,它似乎与教科书中标注的轨道填充顺序不符(教科书中标注的轨道填充顺序如下):
1s
2s 2p
3s 3p
4s 3d 4p
5s 4d 5p
6s 4f 5d 6p
7s 5f 6d 7p
Why [ Kr ] 4 d 10 5 s 2 5 p 5 [\text{Kr}] 4d^{10} 5s^2 5p^5 [Kr]4d105s25p5 is Iodine’s Electron Configuration
为何 [ Kr ] 4 d 10 5 s 2 5 p 5 [\text{Kr}] 4d^{10} 5s^2 5p^5 [Kr]4d105s25p5 是碘的电子构型
If we apply the Aufbau principle to the orbitals above, we would predict that the 5s orbital, because it has lower energy than the 4d orbitals, will fill with electrons before the 4d orbitals do – as you’ve said.
正如您所指出的,如果我们将泡利不相容原理(Aufbau principle)应用于上述轨道,会得出这样的预测:由于 5s 轨道的能量低于 4d 轨道,电子会先填满 5s 轨道,再填充 4d 轨道。
In fact, when two electrons are present in the 5s orbital the energy of the 4d orbitals falls below the energy of 5s. Therefore, the correct configuration for iodine is [ Kr ] 4 d 10 5 s 2 5 p 5 [\text{Kr}] 4d^{10} 5s^2 5p^5 [Kr]4d105s25p5
但实际情况是,当 5s 轨道中填入 2 个电子后,4d 轨道的能量会降至 5s 轨道之下。因此,碘原子的正确电子构型应为 [ Kr ] 4 d 10 5 s 2 5 p 5 [\text{Kr}] 4d^{10} 5s^2 5p^5 [Kr]4d105s25p5。
The reason we don’t always get the result we’d expect from applying the Aufbau Principle to orbitals is that when real electrons begin to interact with one another, some shifts in orbital energy levels can take place.
之所以将泡利不相容原理应用于轨道时,得到的结果并非总能符合预期,是因为在实际原子中,电子之间会发生相互作用,这种相互作用可能导致轨道能级发生偏移。
Thanks for the interesting comment Jessica – it may be worth considering whether we continue to show configurations using the principal quantum number convention or whether we show the actual configuration, when it’s known.
再次感谢您提出的富有洞察力的留言,杰西卡——我们或许值得思考:未来在展示电子构型时,是继续遵循主量子数相关惯例,还是在已知实际构型的情况下,直接展示实际构型。
Principal Energy Levels and Sublevels
主能量级和亚层
能量级、亚层和原子轨道类型的图表
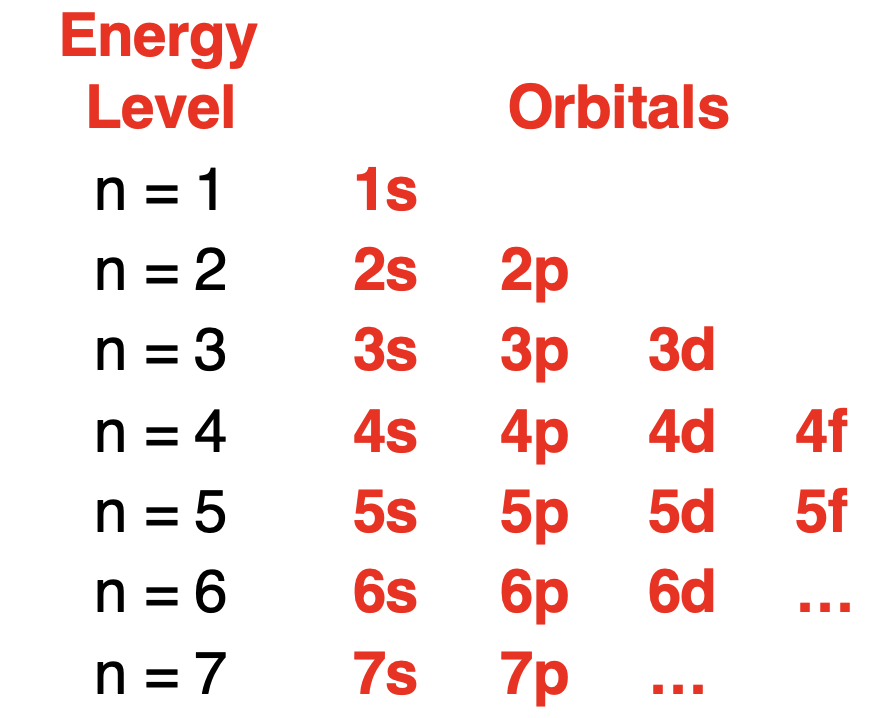
As discussed earlier in this lesson,the Quantum Mechanical Model of the atom proposes that electrons are located in regions of space known as orbitals.Orbitals have precisely known energy (relatively speaking).The principal quantum number (n) determines the principal energy of the orbitals.There are four orbital types of importance – s orbitals,p orbitals,d orbitals,and f orbitals.Each orbital type is considered an energy sublevel within the principal energy level.The first principal energy level has one orbital type – s orbitals.The second principal energy level has two orbital types – s and p orbitals.The s orbital has a lower energy sublevel than the p orbital.The third principal energy level has three orbital types – s,p,and d orbitals.The energy sublevel of the s orbital is lower than the p orbital which is lower than the d orbital.The energy sublevels for the first four energy levels,ordered by energy,are shown below.
正如本课程前面所讨论的,原子的量子力学模型提出,电子位于称为轨道的空间区域内。轨道具有精确已知的能量(相对而言)。主量子数(n)决定了轨道的主能量。有四种重要的轨道类型——s 轨道、p 轨道、d 轨道和 f 轨道。每种轨道类型都被视为主能量级内的一个能量亚级。第一主能量级有一种轨道类型——s 轨道。第二主能量级有两种轨道类型——s 和 p 轨道。s 轨道的能量亚级低于 p 轨道。第三主能量级有三种轨道类型——s、p 和 d 轨道。s 轨道的能量亚级低于 p 轨道,而 p 轨道的能量亚级又低于 d 轨道。下面显示了按能量顺序排列的前四个能量级的能量亚级。
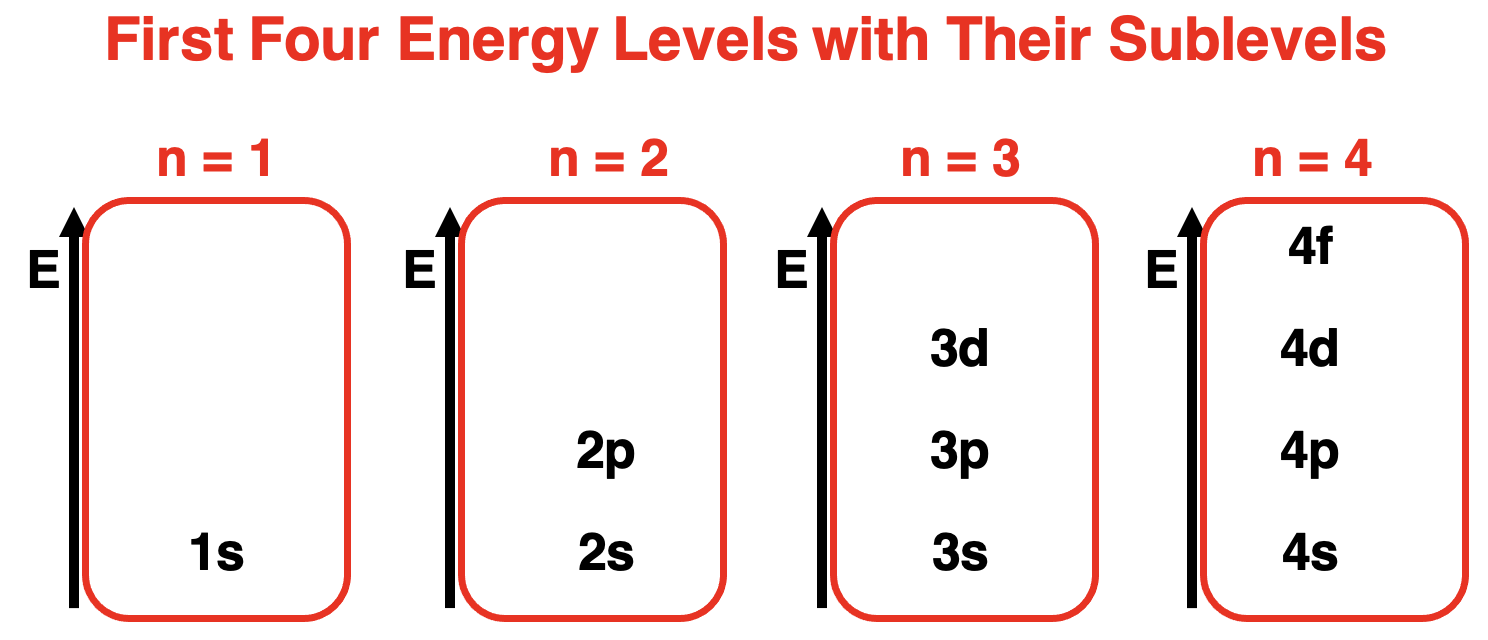
按能量顺序标记轨道类型的前 4 个能量级的图表
Ordering of Atomic Orbitals by Energy
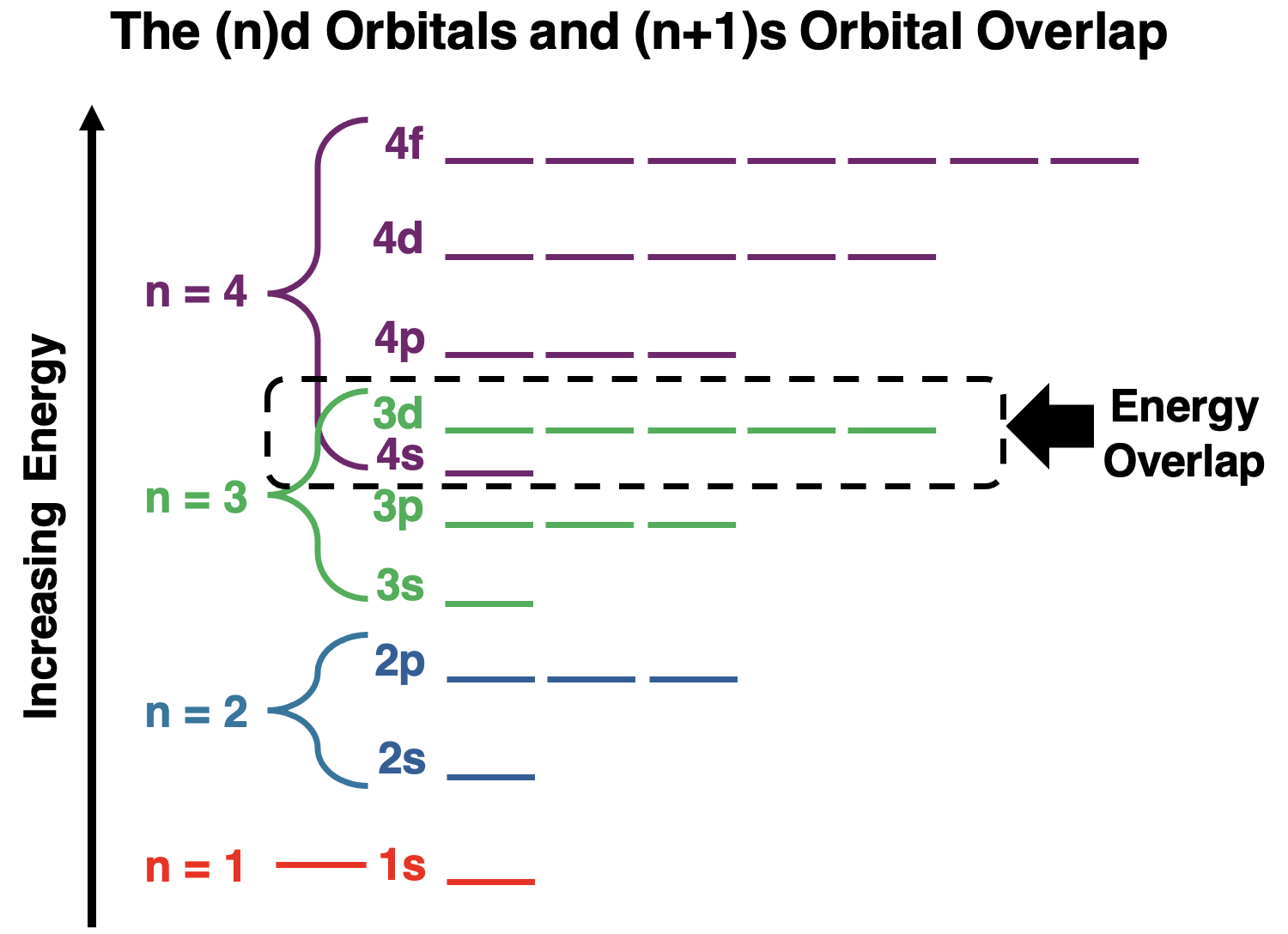
按能量排列原子轨道
Plotting the energy sublevels for the various principal energy levels on the same energy chart yields some surprising results.There is some overlap of energy between consecutive principal energy levels.The overlap is noticeable for any principal energy level containing d orbitals.The s orbital of the next highest energy level is lower in energy than the d orbitals of the previous energy level.As an example,the 4s orbital has lower energy than the 3d orbitals.And the 5s orbital has a lower energy than the 4d orbitals.The pattern continues for other energy levels like those containing the 5d and the 6d orbitals.
将不同主能量级的能量亚级绘制在同一能量图上,会得到一些令人惊讶的结果。连续主能量级之间存在一些能量重叠。对于任何包含 d 轨道的主能量级,这种重叠都是显而易见的。下一个最高能量级的 s 轨道的能量低于前一个能量级的 d 轨道的能量。例如,4s 轨道的能量低于 3d 轨道的能量。5s 轨道的能量低于 4d 轨道的能量。这种模式在包含 5d 和 6d 轨道的其他能量级中也继续存在。
显示第 3 和第 4 能量级重叠的轨道能量的能量级图
A similar surprise is observed for those energy levels containing f orbitals.The 6s and 5p orbitals are lower in energy than the 4f orbitals.And the 7s and 6p orbitals are lower in energy than the 5f orbitals.
对于包含 f 轨道的能量级,也观察到了类似的意外。6s 和 5p 轨道的能量低于 4f 轨道的能量。7s 和 6p 轨道的能量低于 5f 轨道的能量。
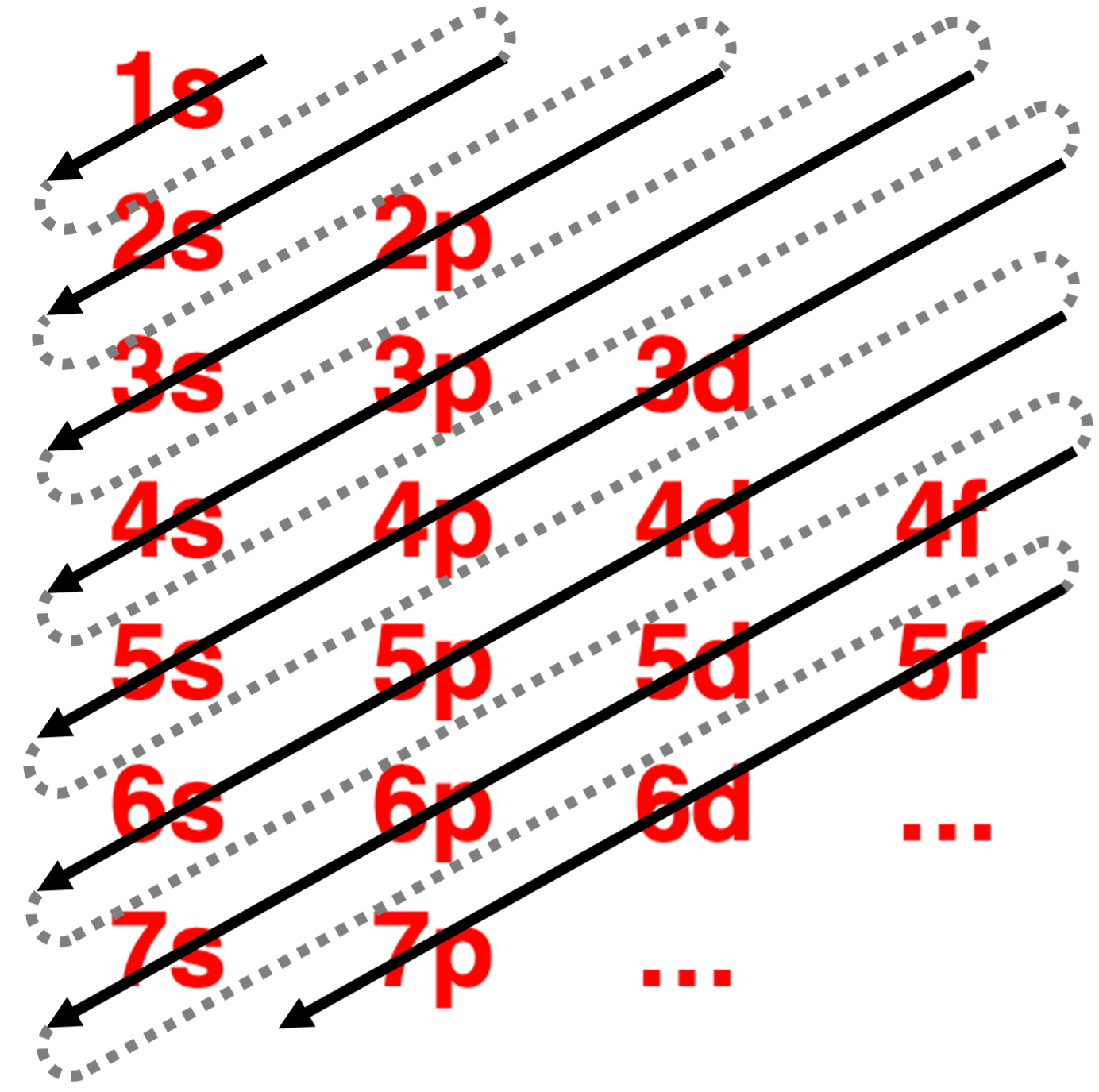
These surprises in the ordering of energy of orbitals can become quite confusing.Predicting which orbitals are lower in energy can get become memory intensive.Fortunately,there are a couple of tricks for remembering the order.The first is presented in the animation below.(The second trick will be presented in Lesson 3.) Begin by listing the energy sublevels for each of the principal energy levels.Then draw diagonals through the listing as shown.The energy ordering is based on which orbitals are reached first by the set of diagonal lines.Study the animation carefully and see if you can repeat it yourself.
这些轨道能量顺序的意外可能会变得相当令人困惑。预测哪些轨道的能量较低可能会变得相当费脑。幸运的是,有一些技巧可以记住顺序。第一个在下面的动画中展示。首先,列出每个主能量级的能量亚级。然后,按照所示的方式画对角线。能量顺序是基于哪组对角线最先到达的轨道。仔细研究动画,看看你是否能自己重复它。

The final result is …
最终结果是……
1s2s2p3s3p4s3d4p5s4d5p6s4f5d6p7s5f6d7p
Describing Electrons and Orbitals in Atoms
描述原子中的电子和轨道
The quantum mechanical model describes electrons as being located in regions of space known as orbitals. There are four different orbital types that are important. Each type has a different shape. Ans orbitalis a spherically shaped orbital. Ap orbitalis a dumbbell shaped orbital with two lobes on the opposite sides of the nucleus. There are also d orbitals and f orbitals with shapes that are difficult to describe in words.
量子力学模型将电子描述为位于称为轨道的空间区域内。有四种重要的不同轨道类型,每种类型都有不同的形状。s 轨道是球形轨道,p 轨道是哑铃形轨道,有两个叶片位于原子核的两侧。此外,还有形状难以用语言描述的 d 轨道和 f 轨道。
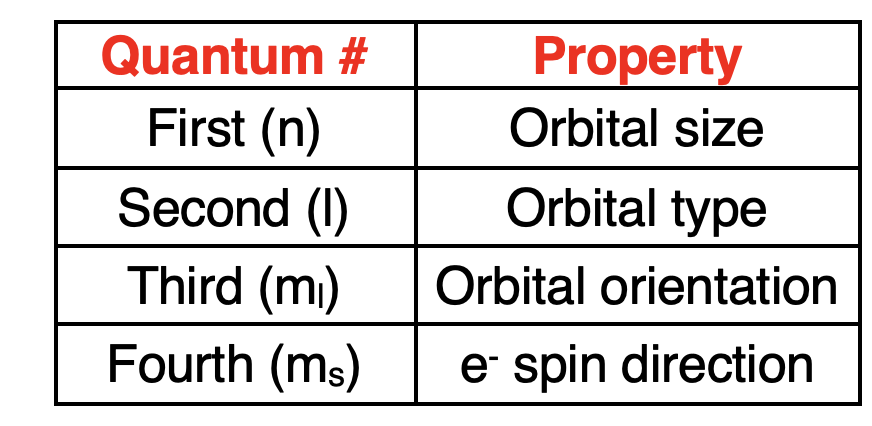
Each electron is described by a unique set of four quantum numbers. The first three quantum numbers define the size, shape, orientation, and energy associated with the orbital. The fourth quantum number describes the spin direction of the electron.
每个电子都由一组独特的四个量子数来描述。前三个量子数定义了轨道的大小、形状、取向和能量。第四个量子数描述了电子的自旋方向。
Principal Quantum Number
主量子数
The first quantum number –n– is known as theprincipal quantum number. It has integer values of 1, 2, 3, etc. The principal quantum number describes the principal energy level of the electron. An electron with an
n
n
n value of 1 is said to be in the first energy level. An electron with an
n
n
n value of 2 is said to be in the second energy level. The energy level of the electron increases as the value of
n
n
n increases. (The second quantum number –l– has a smaller effect upon the energy level.)
第一个量子数 ——n—— 被称为主量子数。它的整数值为 1、2、3 等。主量子数描述了电子的主能量层。具有
n
n
n 值为 1 的电子被认为处于第一能量层。具有
n
n
n 值为 2 的电子被认为处于第二能量层。随着
n
n
n 值的增加,电子的能量层也增加。(第二个量子数 ——l—— 对能量层的影响较小。)
There can be several sets of orbitals having the same
n
n
n value. The number of types of orbitals for any given energy level is equal to the principal quantum number. The first energy level (
n
=
1
n=1
n=1) contains only s orbitals. The second energy level (
n
=
2
n=2
n=2) contains two types of orbitals – s orbitals and p orbitals. The third energy level (
n
=
3
n=3
n=3) contains three types of orbitals - s orbitals, p orbitals, and d orbitals. And the fourth energy level (
n
=
4
n=4
n=4) has all four orbital types.
可以有几组轨道具有相同的
n
n
n 值。任何给定能量层的轨道类型数量等于主量子数。第一能量层(
n
=
1
n=1
n=1)只包含 s 轨道。第二能量层(
n
=
2
n=2
n=2)包含两种类型的轨道 ——s 轨道和 p 轨道。第三能量层(
n
=
3
n=3
n=3)包含三种类型的轨道 ——s 轨道、p 轨道和 d 轨道。第四能量层(
n
=
4
n=4
n=4)包含所有四种轨道类型。
The principal quantum number also affects the size of the orbitals. An s orbital at the
n
=
1
n=1
n=1 energy level is smaller than an s orbital at the
n
=
2
n=2
n=2 energy level. And an s orbital at the
n
=
2
n=2
n=2 energy level is smaller than an s orbital at the
n
=
3
n=3
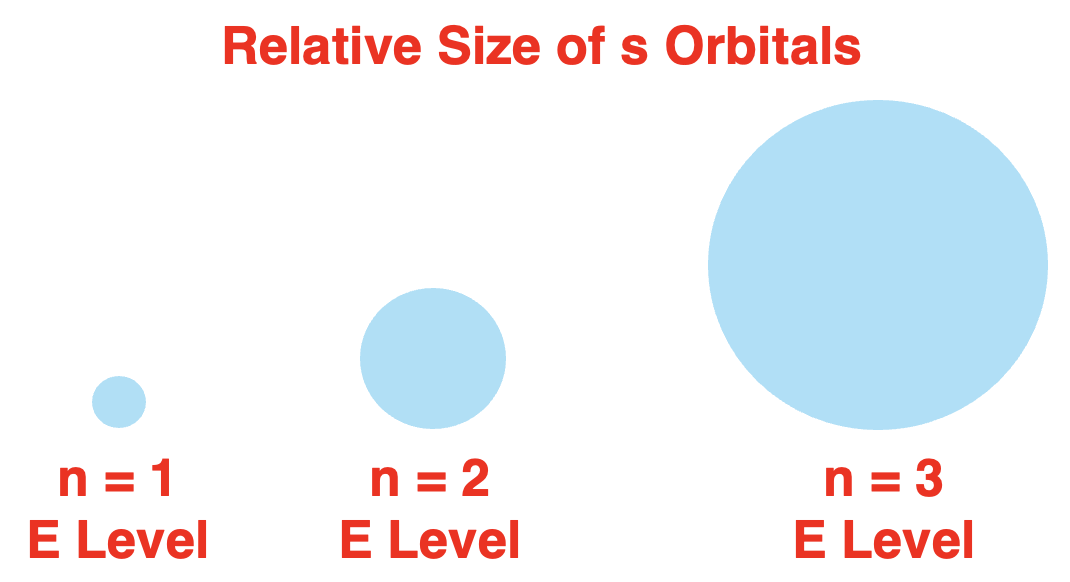
n=3 energy level. This is depicted in the diagram. The same pattern is observed of other orbital types.
主量子数也会影响轨道的大小。在
n
=
1
n=1
n=1 能量层的 s 轨道比在
n
=
2
n=2
n=2 能量层的 s 轨道小。在
n
=
2
n=2
n=2 能量层的 s 轨道比在
n
=
3
n=3
n=3 能量层的 s 轨道小。这一情况在图表中有所展示。其他轨道类型也观察到了相同的模式。
The s Orbital
s 轨道
As mentioned above, every energy level has an s orbital. The s orbital is a spherical orbital. There is one s orbital at each energy level. It is the lowest energy sublevel for every principal energy level. The s orbital of the first energy level is referred to as the 1s orbital. The s orbital of the second energy level is referred to as the 2s orbital. Preceding the orbital type (“s”) with the principal quantum number (1, 2, 3, etc.) is common notation in quantum mechanics.
如上所述,每个能量层都有一个 s 轨道。s 轨道是球形轨道。每个能量层都有一个 s 轨道。它是每个主能量层的最低能量亚层。第一能量层的 s 轨道称为 1s 轨道。第二能量层的 s 轨道称为 2s 轨道。在轨道类型(“s”)之前加上主量子数(1、2、3 等)是量子力学中的常见表示法。
The p Orbitals
p 轨道
The p orbitals are found in all energy levels with a principal quantum number of 2 or higher. The p orbitals are described as dumbbell shaped orbitals. There are two lobes positioned on opposite sides of the nucleus. To help remember theporbital shape, we call thempinched cylinders (with bulging ends). There are three p orbitals at each of these energy levels. They are distinguished by their orientation relative to the imaginary x-y-z axes and sometimes termed px, py, and pz orbitals. The p orbitals of the second energy level are referred to as the 2p orbitals. The p orbitals of the third energy level are referred to as the 3p orbitals.
p 轨道存在于主量子数为 2 或更高的所有能量层中。p 轨道被描述为哑铃形轨道。有两个叶片位于原子核的两侧。为了帮助记忆p轨道的形状,我们称它们为p形圆柱(两端膨胀)。在这些能量层中各有三个 p 轨道。它们根据相对于假想的 x-y-z 轴的取向而被区分开来,有时被称为 px、py 和 pz 轨道。第二能量层的 p 轨道称为 2p 轨道。第三能量层的 p 轨道称为 3p 轨道。
The d Orbitals
d 轨道
The d orbitals are found in all energy levels with a principal quantum number of 3 or higher. There are five d orbitals at each of these energy levels. The shapes of the d orbitals are rather complex. Four of the d orbitals could be described as having a set of four lobes arranged perpendicular to each other. The fifth d orbital looks like a p orbital with a ring around its center. Most introductory Chemistry courses do not require any knowledge of their shapes. The d orbitals of the third energy level are referred to as the 3d orbitals. The d orbitals of the fourth energy level are referred to as the 4d orbitals.
d 轨道存在于主量子数为 3 或更高的所有能量层中。在这些能量层中各有五个 d 轨道。d 轨道的形状相当复杂。其中四个 d 轨道可以被描述为有一组四个叶片,它们相互垂直排列。第五个 d 轨道看起来像一个中心带环的 p 轨道。大多数入门化学课程并不要求了解它们的形状。第三能量层的 d 轨道称为 3d 轨道。第四能量层的 d 轨道称为 4d 轨道。
The f Orbitals
f 轨道
The f orbitals are found in all energy levels with a principal quantum number of 4 or higher. There are seven f orbitals at each of these energy levels. Like the d orbitals, their shapes are rather complex and typically not a required understanding in most introductory Chemistry courses. The f orbitals of the fourth energy level are referred to as the 4f orbitals.
f 轨道存在于主量子数为 4 或更高的所有能量层中。在这些能量层中各有七个 f 轨道。与 d 轨道类似,它们的形状相当复杂,通常在大多数入门化学课程中并不要求理解。第四能量层的 f 轨道称为 4f 轨道。
Electron Shells
电子层
The collection of orbitals located in each energy level make up what is sometimes referred to as anelectron shell. For instance, the second energy level contains both s orbitals and p orbitals. There are three p orbitals with one lying along each axis. These four orbitals (the one s and three p orbitals) combine to form the
n
=
2
n=2
n=2 electron shell or the second electron shell.
每个能量层中的轨道集合有时被称为一个电子层。例如,第二能量层包含 s 轨道和 p 轨道。有三个 p 轨道,每个轨道分别位于一个轴上。这四个轨道(一个 s 轨道和三个 p 轨道)组合形成了
n
=
2
n=2
n=2 电子层或第二电子层。

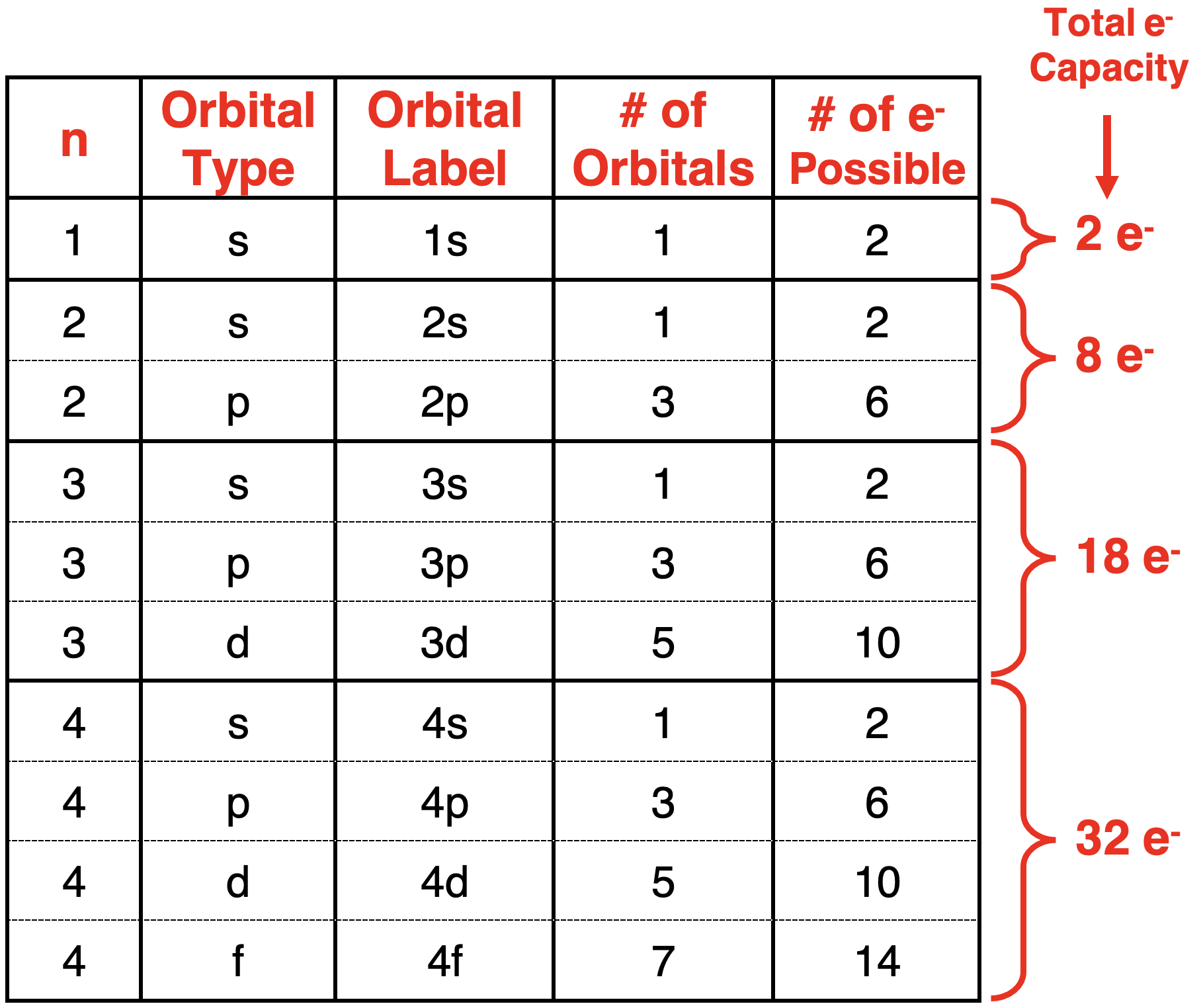
Patterns for the Number of Orbitals and Electron Capacity
轨道数量和电子容量的模式
As inferred in the above discussion, there are clear patterns that emerge from the mathematical solutions of the Schrodinger equation. These patterns determine what orbital types are present at each energy level, how many of those orbitals are present, and the number of electrons that can be in those orbitals. The patterns are organized into the following table.
正如在上述讨论中推断的那样,从 薛定谔方程 的数学解中可以得出清晰的 * 模式 *。这些模式决定了每个能量层中存在的轨道类型、这些轨道的数量以及这些轨道中可以容纳的电子数量。这些模式被整理在以下表格中。
What Is the Wave-Mechanical Model?
波动力学模型是什么?

Louis de Broglie’s treatment of the electron as a wave was the precursor to the modern model of the atom.
路易・德布罗意将电子视为一种波的处理方式是现代原子模型的先驱。
Referred to as theWave Mechanical Model, or more commonly theQuantum Mechanical Model, the modern model is a highly mathematical model that describes electrons by a wave function,Ψ.
现代模型被称为波动力学模型,更常见的是量子力学模型,它是一个高度数学化的模型,通过一个 * 波函数 *Ψ来描述电子。
Proposed by Austrian physicist Erwin Schrodinger in 1926, the wave mechanical model is a complex mathematical description of the atom and its electrons.
该模型由奥地利物理学家埃尔温・薛定谔于 1926 年提出,是对原子及其电子的复杂数学描述。
The x-dimension version of theSchrodinger wave equationis shown at the right. There is also a y-dimension and z-dimension equation.
右侧展示的是薛定谔波动方程的 x 方向版本。还有 y 方向和 z 方向的方程。
Given the complexity of the mathematics, we will avoid the equations and instead discuss the conclusions that were derived from the use of the equations to describe the electrons.
鉴于数学的复杂性,我们将避免涉及方程,而是讨论从这些方程中得出的关于电子的结论。
Schrodinger’s approach to modeling the atom was to specify the location of each electron by a probability function.
薛定谔对原子建模的方法是通过概率函数来确定每个电子的位置。
Instead of locating the electron at a point, Schrodinger described the electron as being located withinorbitals.
他没有将电子定位在某一点上,而是描述电子位于轨道内。
An orbital isnotan orbit.
轨道不是轨道。
An orbit describes the electron moving along a well-defined path a precise distance from the nucleus.
轨道描述的是电子沿着一条明确的路径在距离原子核的精确距离上运动。
An orbital describes a region of space in which there is a 90% probability that the electron will be located.
轨道描述的是一个空间区域,在这个区域内有 90% 的概率可以找到电子。
There is no effort to describe where the electron is within the space, nor how it arrived at its current location, nor which direction it is heading and where it will be next.
并没有尝试描述电子在空间中的具体位置,也没有描述它是如何到达当前位置的,以及它将朝哪个方向运动,下一步会出现在哪里。
The Wave Mechanical Model describes electrons as having a high likelihood of being in an orbital that has a (relatively) precisely known energy.
波动力学模型将电子描述为有很高的可能性出现在一个具有(相对)精确能量的轨道上。
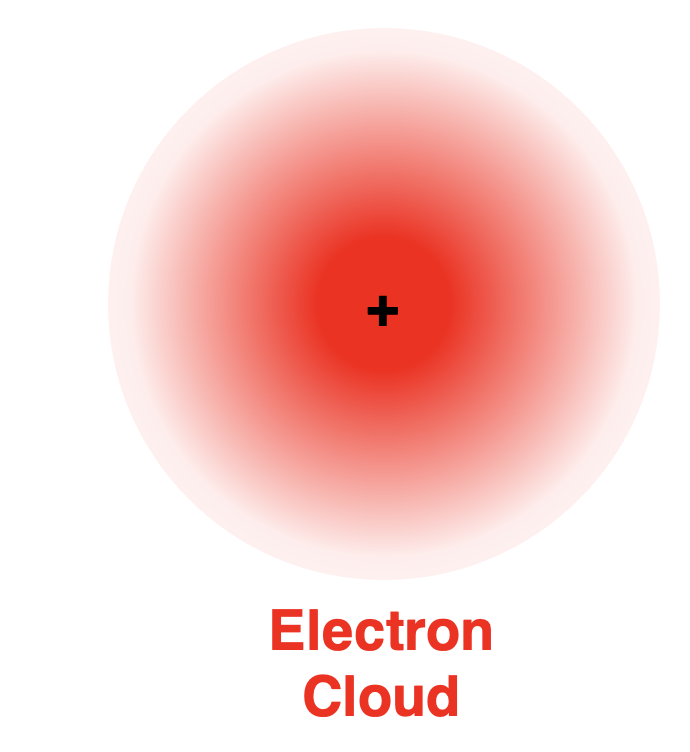
These orbitals are often plotted in three dimensions as anelectron cloud.
这些轨道通常在三维空间中以电子云的形式绘制出来。
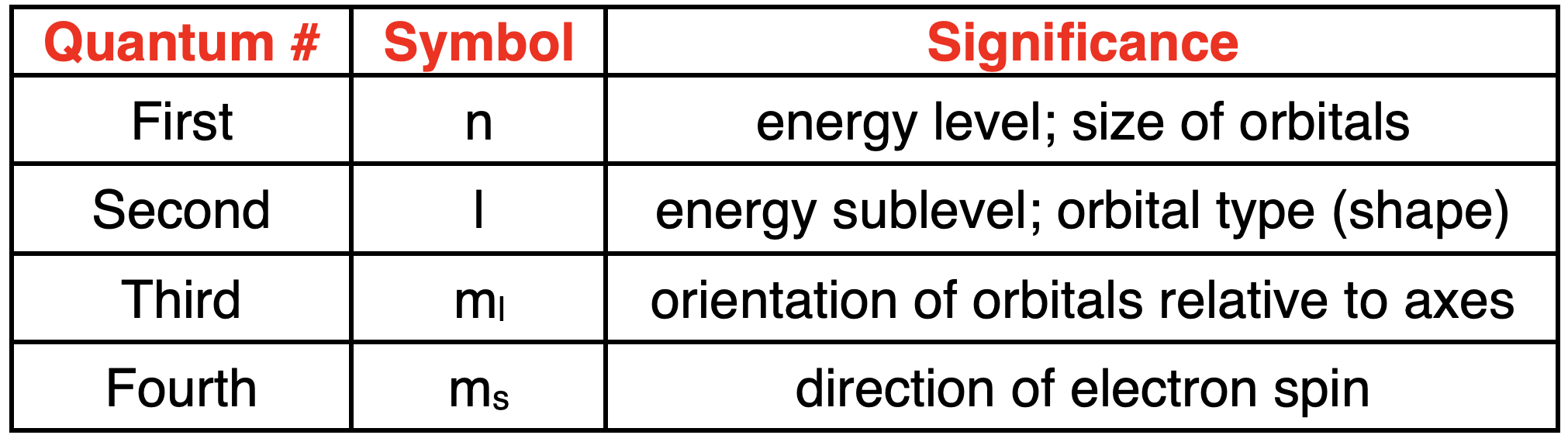
The Four Quantum Numbers
四个量子数
The solutions to the Schrodinger wave equation result in four quantum numbers that describe the orbitals and the electrons that are inside of them.
薛定谔波动方程的解产生了四个量子数,用于描述轨道以及其中的电子。
Each electron in the atom has its own unique set of four quantum numbers.
原子中的每个电子都有自己独特的一组四个量子数。
The quantum numbers and their significance are described below:
以下是对这些量子数及其意义的描述:
First Quantum NumbernPrincipal Energy Level
第一量子数 n 主能量层
The energy level of an electron depends mostly on the first quantum number.
电子的能量层主要取决于第一量子数。
Values for this number are whole numbers beginning with 1.
这个数的取值是从 1 开始的整数。
The energy level is greater for greater values of
n
n
n.
n
n
n 的值越大,能量层越高。
The energy of an electron is affected to a lesser degree by the second quantum number.
电子的能量受第二量子数的影响较小。
The value of
n
n
n also affects the size of the orbitals. Orbitals are larger for larger values of
n
n
n.
n
n
n 的值还会影响轨道的大小。
n
n
n 越大,轨道越大。
Second Quantum NumberlOrbital Type/Shape
第二量子数 l 轨道类型 / 形状
Every principal energy level consists of one or more types of orbitals.
每个主能量层都包含一种或多种类型的轨道。
The different types of orbitals have unique shapes.
不同类型的轨道具有独特的形状。
When
l
l
l has a value of 0, the orbital type is spherical (s orbital).
当
l
l
l 的值为 0 时,轨道类型为球形(s 轨道)。
When
l
l
l has a value of 1, the orbital type can be described as a dumbbell with two lobes on opposite sides of the nucleus (p orbital).
当
l
l
l 的值为 1 时,轨道类型可以描述为一个哑铃形,两个叶片位于原子核的两侧(p 轨道)。
There are alsod orbitaltypes andf orbitaltypes. Their shapes are more complex.
还有 d 轨道类型和 f 轨道类型。它们的形状更为复杂。
Different orbital types in the same principal energy level will have slightly different energies.
同一主能量层中的不同轨道类型将具有略微不同的能量。
Thus the orbital types are often referred to asenergy sublevels.
因此,轨道类型通常被称为能量亚层。
While thenquantum number is the main factor affecting the energy level, thelquantum number has a small influence, affects the energy sublevel.
虽然 n 量子数是影响能量层的主要因素,但l量子数也有一定的影响,它影响能量亚层。
The s-type orbitals are lower in energy than the p-type orbitals. The p-type orbitals are lower in energy than the d-type orbitals which are lower in energy than the f-type orbitals.
s 型轨道的能量低于 p 型轨道,p 型轨道的能量低于 d 型轨道,d 型轨道的能量低于 f 型轨道。
Third Quantum Number
第三量子数
ml Orbital Orientation
ml 轨道方向
The s-orbitals are symmetrical about the origin in all directions. There is only one way by which it can be oriented.
s 轨道在所有方向上都关于原点对称。它只有一种可能的取向。
There are three different p-orbital types. They each consist of three lobes. The lobes are aligned along one of the three axes.
有三种不同的 p 轨道类型。每种 p 轨道都包含三个叶片,这些叶片分别沿着三个坐标轴之一排列。
The third quantum number indicates the axis along which the lobes are aligned.
第三量子数表示叶片所对齐的坐标轴。
The d orbitals and f orbitals also have specific orientations relative to the axes. The third quantum number describes their orientation of the orbitals.
d 轨道和 f 轨道也相对于坐标轴有特定的取向。第三量子数描述了轨道的取向。
Fourth Quantum Number
第四量子数
ms Electron Spin
ms 电子自旋
The first three quantum numbers describe the relative size, type, and orientation of the orbitals. These orbitals are the region of space in which the electrons are located.
前三个量子数描述了轨道的相对大小、类型和取向。这些轨道是电子所在的空间区域。
There can be at most two electrons in any one of the orbitals. The fourth quantum number describes the electrons.
每个轨道最多可以容纳两个电子。第四量子数描述了电子。
Two electrons in the same orbital are distinguished from one another by their spin direction – clockwise or counter-clockwise.
同一轨道中的两个电子通过它们的自旋方向 —— 顺时针或逆时针 —— 来区分彼此。
Summary of the Four Quantum Numbers
四个量子数的总结
Here is a summary of the four quantum numbers:
以下是四个量子数的总结:
Examples of Quantum Numbers
量子数的例子
You may be curious and wondering: what would be an example of the set of quantum numbers for a ground state electron in an atom? If you are curious, here’s a few examples:
你可能会好奇并想知道:一个原子中处于 * 基态 * 的电子的量子数集合是什么样的?如果你好奇的话,这里有一些例子:
For the one electron in the hydrogen atom, the set of four quantum numbers might be:
对于氢原子中的一个电子,其四个量子数的集合可能是:
n , l , m l , m s = 1 , 0 , 0 , + 1 2 n, l, m_l, m_s = 1, 0, 0, +\frac {1}{2} n,l,ml,ms=1,0,0,+21
For two electrons in the helium atom, the set of four quantum numbers might be:
对于氦原子中的两个电子,其四个量子数的集合可能是:
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
+
1
2
n, l, m_l, m_s = 1, 0, 0, +\frac {1}{2}
n,l,ml,ms=1,0,0,+21 and
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
−
1
2
n, l, m_l, m_s = 1, 0, 0, -\frac {1}{2}
n,l,ml,ms=1,0,0,−21
For three electrons in the lithium atom, the set of four quantum numbers might be:
对于锂原子中的三个电子,其四个量子数的集合可能是:
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
+
1
2
n, l, m_l, m_s = 1, 0, 0, +\frac {1}{2}
n,l,ml,ms=1,0,0,+21 and
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
−
1
2
n, l, m_l, m_s = 1, 0, 0, -\frac {1}{2}
n,l,ml,ms=1,0,0,−21 and
n
,
l
,
m
l
,
m
s
=
2
,
0
,
0
,
+
1
2
n, l, m_l, m_s = 2, 0, 0, +\frac {1}{2}
n,l,ml,ms=2,0,0,+21
For five electrons in the lithium atom, the set of four quantum numbers might be:
对于锂原子中的五个电子,其四个量子数的集合可能是:
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
+
1
2
n, l, m_l, m_s = 1, 0, 0, +\frac {1}{2}
n,l,ml,ms=1,0,0,+21 and
n
,
l
,
m
l
,
m
s
=
1
,
0
,
0
,
−
1
2
n, l, m_l, m_s = 1, 0, 0, -\frac {1}{2}
n,l,ml,ms=1,0,0,−21 and
n
,
l
,
m
l
,
m
s
=
2
,
0
,
0
,
+
1
2
n, l, m_l, m_s = 2, 0, 0, +\frac {1}{2}
n,l,ml,ms=2,0,0,+21 and
n
,
l
,
m
l
,
m
s
=
2
,
0
,
0
,
−
1
2
n, l, m_l, m_s = 2, 0, 0, -\frac {1}{2}
n,l,ml,ms=2,0,0,−21 and
n
,
l
,
m
l
,
m
s
=
2
,
1
,
0
,
+
1
2
n, l, m_l, m_s = 2, 1, 0, +\frac {1}{2}
n,l,ml,ms=2,1,0,+21
Some people claim “Curiosity killed the cat.” We say “Keep being curious! It’s never killed any cats.” After all, nobody knows whether the cat was dead or alive before curiosity opened the box.
有些人声称 “好奇心害死猫”。我们说 “保持好奇心!它从未害死过任何猫。” 毕竟,没有人知道在好奇心打开盒子之前,猫是死是活。
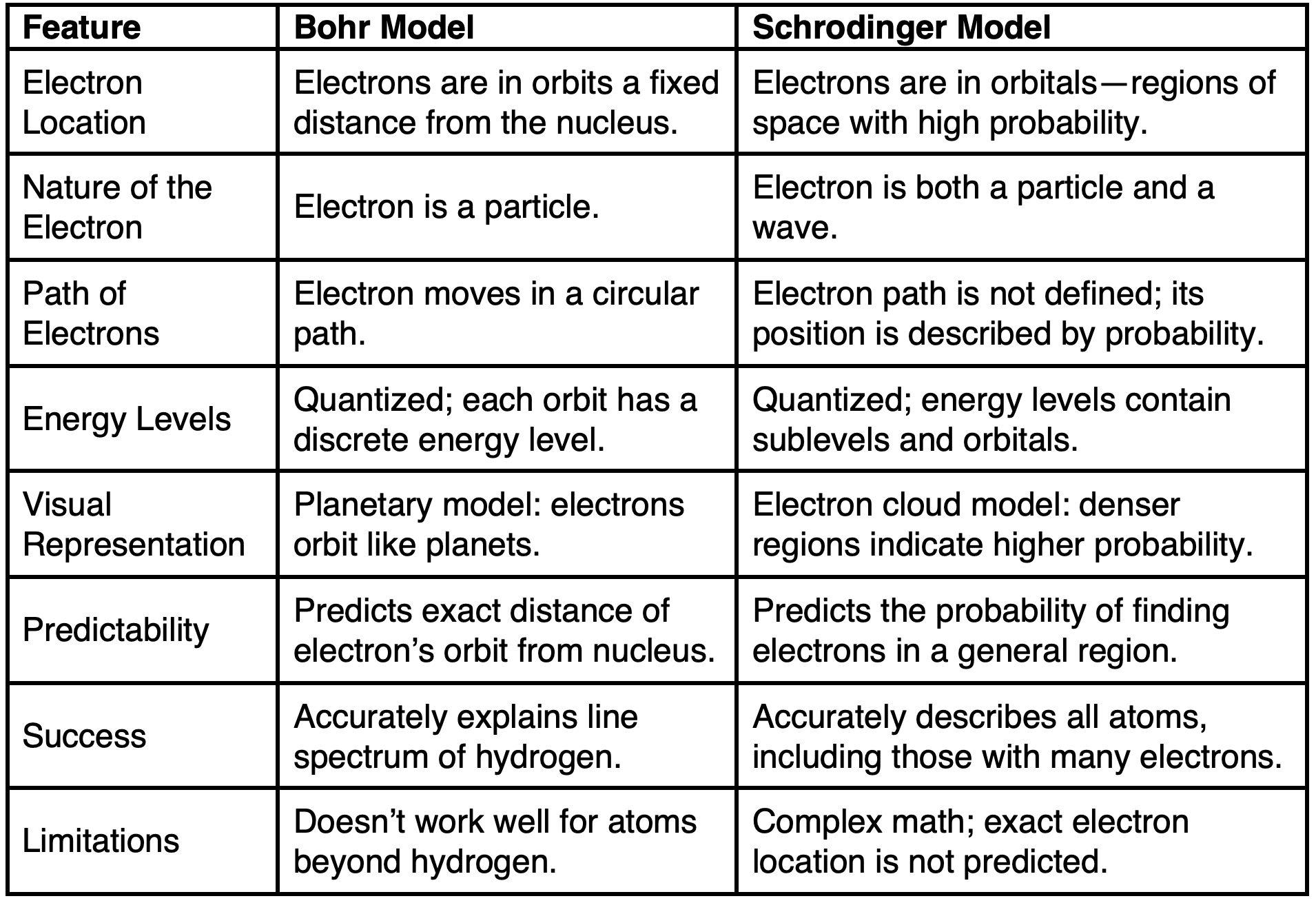
Schrodinger vs. Bohr Atomic Models
薛定谔与玻尔原子模型
The Schrodinger wave mechanical model replaced the Bohr atomic model.
薛定谔的波动力学模型取代了玻尔原子模型。
While both atomic models were quantum models, there were significant differences.
尽管这两种原子模型都是量子模型,但它们之间存在显著的差异。
The table below describes some of those differences.
下表描述了其中一些差异。
Electron Configurations and Chemical Properties
电子配置与化学性质
Chemical properties describe how a substance interacts with other substances to form compounds.
化学性质描述了一种物质与其他物质相互作用形成化合物的方式。
The chemical properties of an element include descriptions of how that element combines with other elements and the ratio of atoms in the resulting compound.
元素的 化学性质 包括该元素与其他元素结合的方式以及在生成化合物中原子的比例。
These chemical properties are determined by the manner in which the configuration of electrons in the atoms of those elements.
这些化学性质是由这些元素的原子中电子的排布方式决定的。
The foundation relies on the use of electron configurations.Electron configurationsare a symbolic means of showing the location of electrons within an atom.
这一基础依赖于电子排布式的使用。电子排布式是一种展示原子中电子位置的符号方法。
Electrons are housed inside of orbitals. An electron configuration identifies the address of all the houses and the number of electrons residing in the houses.
电子被 “安置” 在轨道内。电子排布式确定了所有 “房屋” 的 “地址” 以及居住在这些 “房屋” 中的电子数量。
The orbitals are identified by standard orbital notation (1s, 2s, 2p, 3d, etc.) and a superscript indicates how many electrons are housed inside orbitals of that type.
轨道通过标准轨道符号(1s、2s、2p、3d 等)来识别,上标表示该类型轨道内容纳的电子数量。
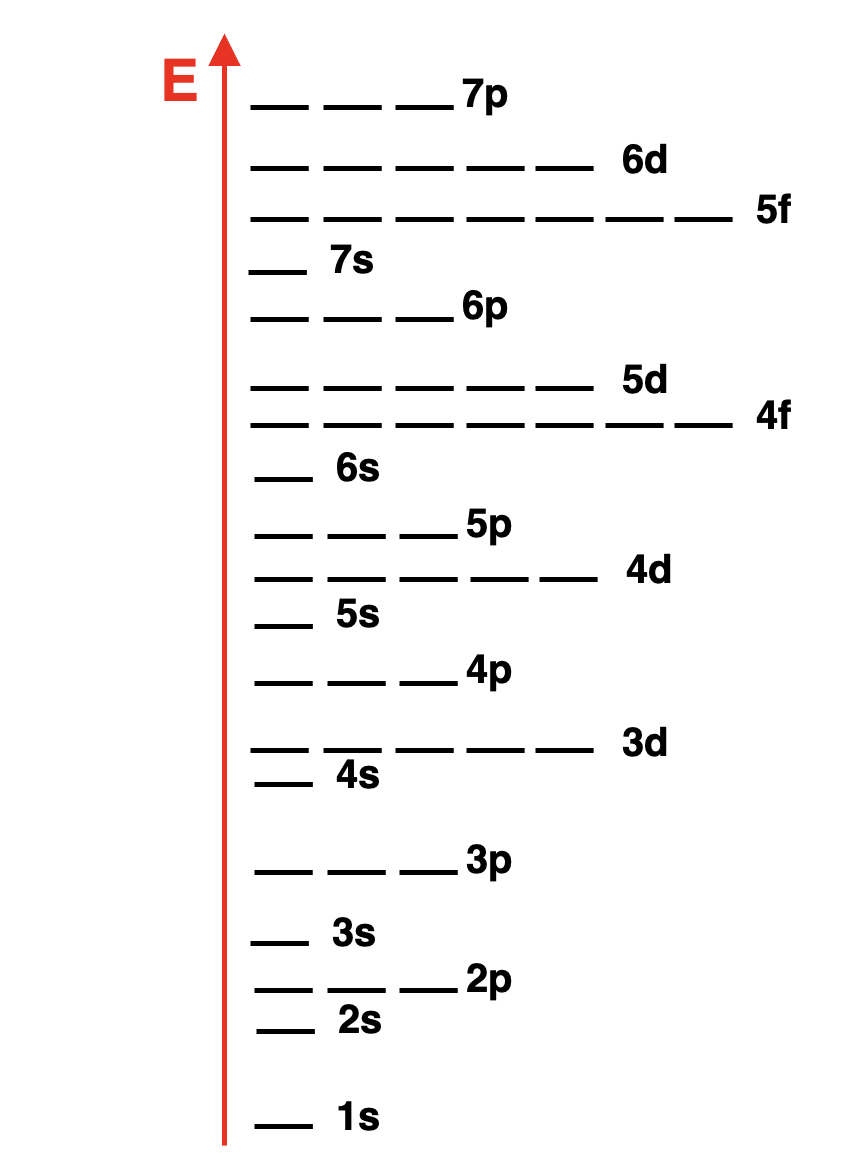
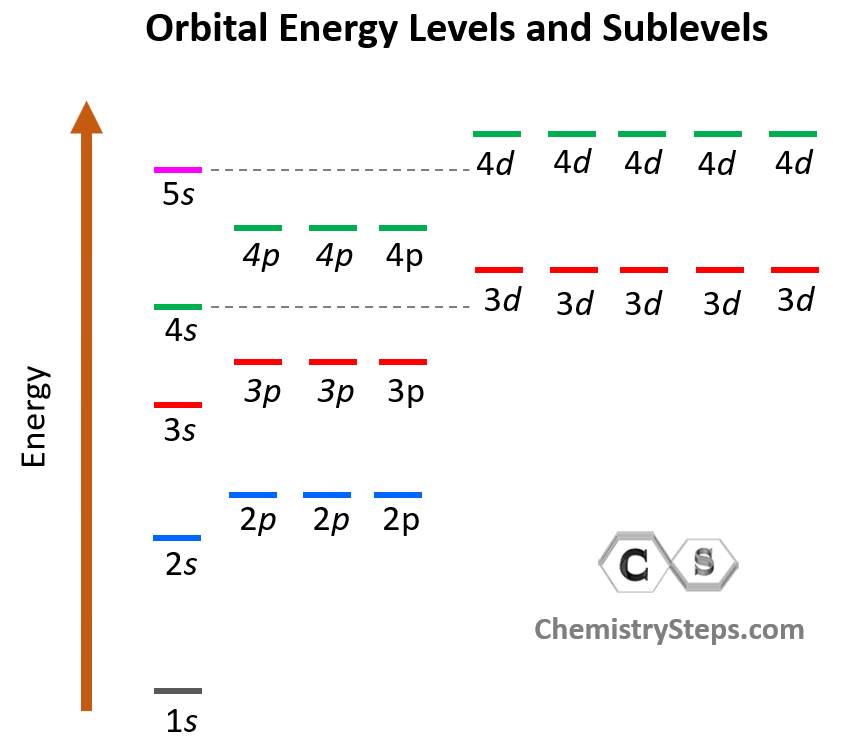
Energy Levels
能量层
To write an electron configuration for an element, one must know the names of the orbitals and their relative energies.
要为一个元素写出电子排布式,必须知道轨道的名称及其相对能量。
The graphic at the right identifies the names (orbital notation) of all the orbitals and places them on a graph to show their relative energy.
右侧的图表标明了所有轨道的 * 名称 *(轨道符号)并将它们放置在图表上以显示它们的相对能量。
Generally, orbitals with a smaller
n
n
n value (the number in the orbital notation) have lower energies.
一般来说,具有较小
n
n
n 值(轨道符号中的数字)的轨道具有较低的能量。
There are some surprises for any principal energy level containing
d
d
d orbitals.
对于任何包含
d
d
d 轨道的主能量层,存在一些意外情况。
The
s
s
s orbitals of the 4th energy level are lower in energy than the
d
d
d orbitals of the 3rd energy level. The same can be said for all other energy levels with an even higher
n
n
n.
第 4 能量层的
s
s
s 轨道的能量低于第 3 能量层的
d
d
d 轨道的能量。对于所有具有更高
n
n
n 值的其他能量层,情况也是如此。
The graph also shows that the 5s, 5p, and 6s orbitals are lower in energy than the 4f orbitals.
图表还显示,5s、5p 和 6s 轨道的能量低于 4f 轨道的能量。
These surprise orderings make the task of ordering the orbitals by energy very memory intensive.
这些意外的排序使得按照能量对轨道进行排序的任务非常依赖记忆。
One means of generating the order involves the use of the diagonal rule (sometimes referred to as the Madelung Rule).
一种生成排序的方法涉及使用对角线规则(有时也称为马德隆规则)。
The orbitals are listed by principal energy level. Then diagonals are drawn through the listing. The use of this rule is depicted in the diagram. More details can be found in Lesson 2c.
轨道按主能量层列出。然后在列表中画出对角线。该规则的使用在图表中有所展示。
Rules for Electron Configurations
电子排布式的规则
Writing electron configurations for elements also demands an understanding of the order by which electrons enter the orbitals.
为元素写出电子排布式还需要了解电子进入轨道的顺序。
This was also thoroughly discussed in Lesson 2c so the discussion here will be brief.
这一内容在第 2 课 c 中也进行了详细的讨论,因此这里将简要讨论。
There are three rules to adhere to when deciding the orbital that the 6th, the 8th, the 14th, the 16th, etc. electron will be housed in.
在决定第 6 个、第 8 个、第 14 个、第 16 个等电子将被 “安置” 在哪个轨道时,需要遵循三条规则。
1.Aufbau Principle: Electrons will completely fill the lowest energy orbitals first before entering orbitals of the next highest energy.
构造原理:电子会先完全填满最低能量的轨道,然后才进入下一个较高能量的轨道。
2.Hund’s Rule: Electrons will half-fill the orbitals of a given energy sublevel with the same spin direction before pairing up inside such orbitals.
洪特规则:电子会先以相同的自旋方向半填满给定能量亚层的轨道,然后才会在这些轨道内成对。
3.Pauli Exclusion Principle: When electrons pair up inside of orbitals, they do so with opposite spin direction.
泡利不相容原理:当电子在轨道内成对时,它们的自旋方向相反。
Orbital box diagrams were used to show the placement of electrons. Electrons are represented by arrows. The direction that they point (up or down) reflects their spin direction.
轨道方框图用于展示电子的放置位置。电子用箭头表示。箭头的指向(向上或向下)反映了它们的自旋方向。
Orbital Box Diagrams and Electron Configurations
轨道方框图与电子排布式
Orbital box diagrams are useful tools for determining electron configurations.
轨道方框图是确定电子排布式的有用工具。
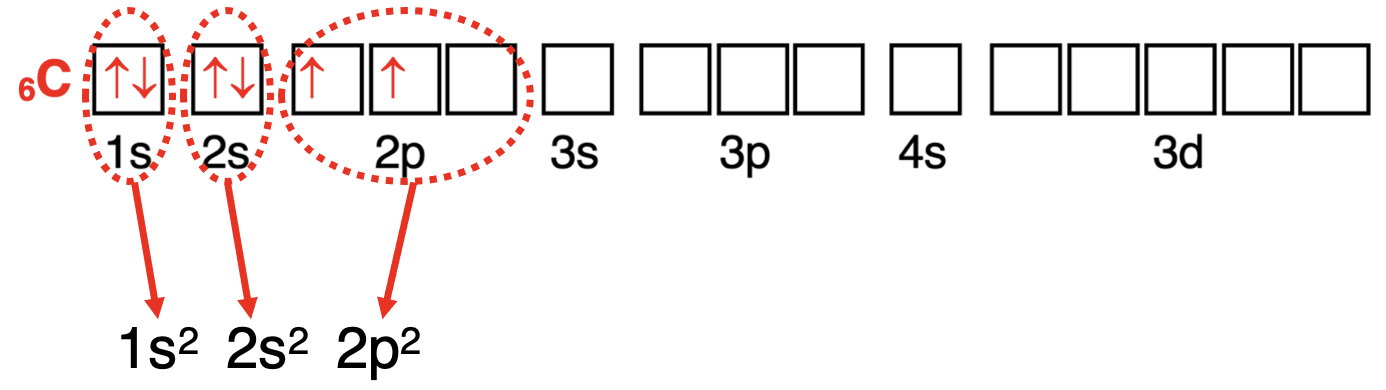
Once the diagram is completed, begin writing the electron configuration. Begin on the left side of the diagram with the lowest energy orbital. Write the orbital notation of the orbital (1s). Add a superscript to indicate the number of electrons in the orbital (1s²). Continue from left (lowest energy) to right (higher energies) across the orbital box diagram. Write the orbital notation plus superscript for any orbital type with electrons. The process is shown for carbon and chlorine below.
完成方框图后,开始写出电子排布式。从方框图的左侧开始,选择最低能量的轨道。写出轨道的符号(1s)。加上上标以表示轨道中的电子数量(1s²)。从左(最低能量)到右(更高能量)依次穿过轨道方框图,为任何有电子的轨道类型写出轨道符号和上标。以下展示了碳和氯的过程。
Examples of Electron Configurations
电子排布式的例子
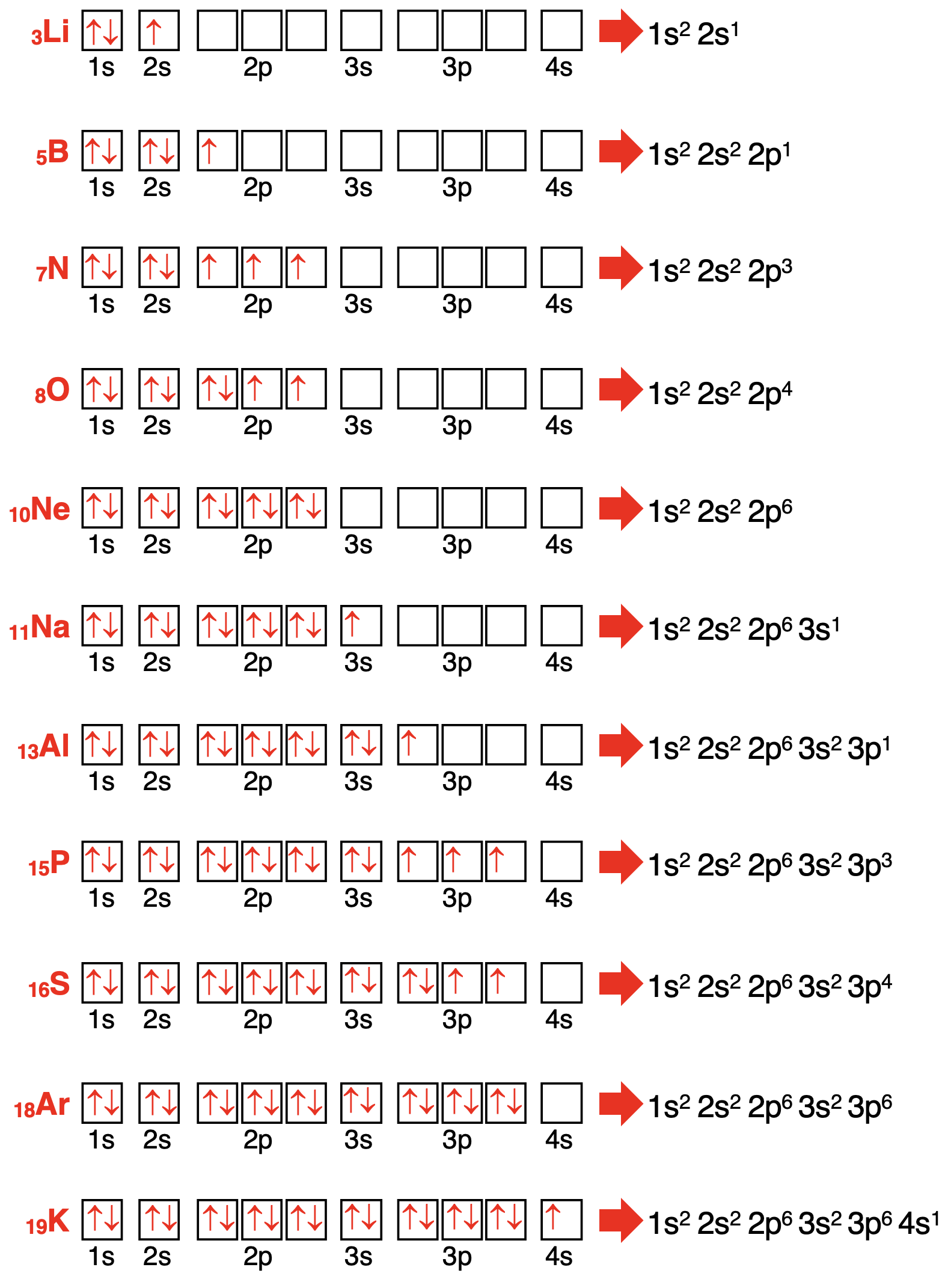
Eleven more examples of electron configurations are shown below. Observe how the orbital box diagram naturally translates into an electron configuration.
以下展示了更多 11 个电子排布式的例子。观察轨道方框图是如何自然地转化为电子排布式的。
Writing Abbreviated Electron Configurations
写出简化的电子排布式
Electron configurations can become quite long. Consider the configuration for barium with 56 electrons:
电子排布式可能会变得很长。以有 56 个电子的钡的排布式为例:
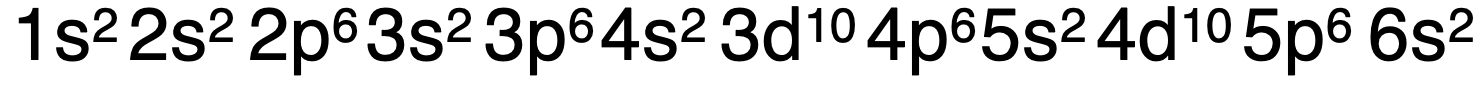
After writing a few barium-like configurations, one begins thinking is there a shortcut? The good news is that there is. The shortcut is to write the so-calledabbreviated electron configuration. Writing the abbreviated form involves the following:
在写出几个类似钡的排布式后,人们会开始思考 * 有没有捷径?* 好消息是确实有。捷径就是写出所谓的简化电子排布式。写出简化形式包括以下步骤:
-
Locate the element on the periodic table.
在周期表中找到该元素的位置。 -
Identify the noble gas element (Group 18) at the end of the row above the element.
确定该元素所在行上方行末的稀有气体元素(第 18 族)。 -
Write the symbol of the noble gas enclosed in brackets. That takes care of all the electrons included in the noble gas. For instance, if you write [Xe], you are accounting for the first 54 electrons.
写出稀有气体的符号,并将其括在方括号内。这将涵盖稀有气体所包含的所有电子。例如,如果你写出 [Xe],那么你就已经考虑了前 54 个电子。 -
Write the remainder of the electron configuration, beginning with the electron after the noble gas’s last electron. For the abbreviated electron configuration of barium, write [Xe]. Then add the location of the 55th and 56th electrons.
写出剩余的电子排布式,从稀有气体最后一个电子之后的电子开始。对于钡的简化电子排布式,写出 [Xe],然后加上第 55 个和第 56 个电子的位置。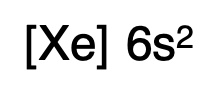
To use the shortened configuration, one must know either the orbital notation for the element at the end of the row or the orbital notation for the element at the beginning of a row. This is shown below. (We will have more to say about it in Lesson 3b.)
要使用简化排布式,必须知道行末元素的轨道符号或行首元素的轨道符号。以下进行了展示。
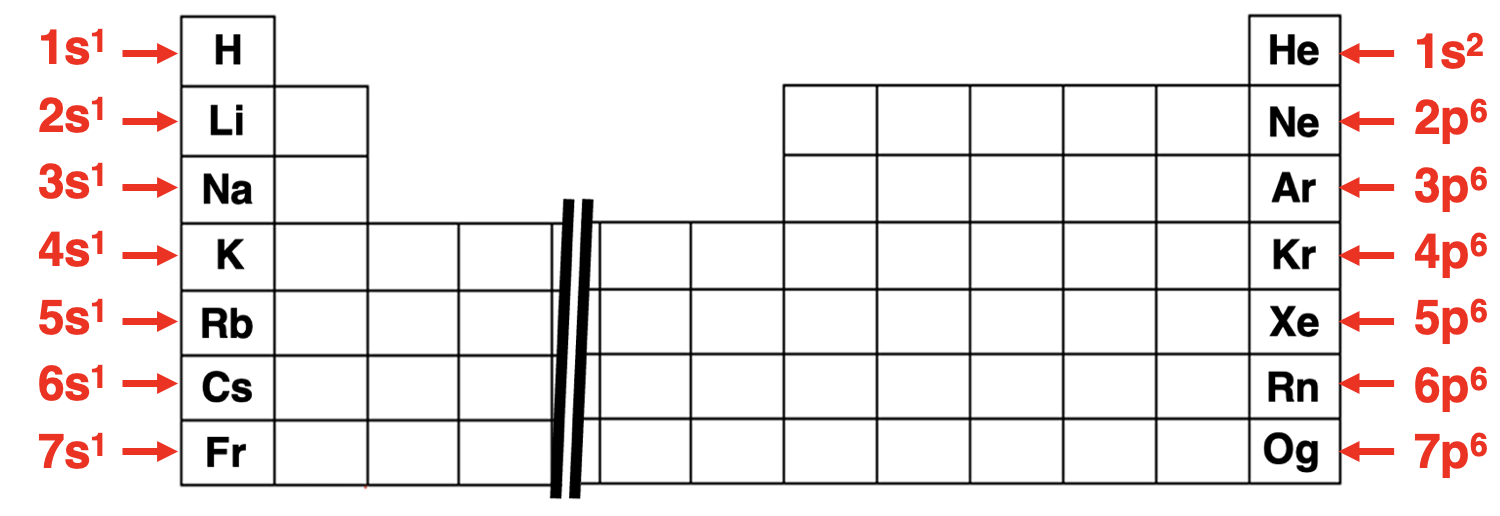
The table below shows the abbreviated electron configuration for a variety of elements. Notice that they always begin with the symbol of a noble gas element enclosed in brackets. Once that is written, the energy level diagram showing the ordering of orbitals is used to finish out the configuration in the usual manner.
下表展示了多种元素的简化电子排布式。注意它们总是以方括号内的稀有气体元素符号开头。一旦写好,就使用显示轨道顺序的能量层图,以通常的方式完成排布式。
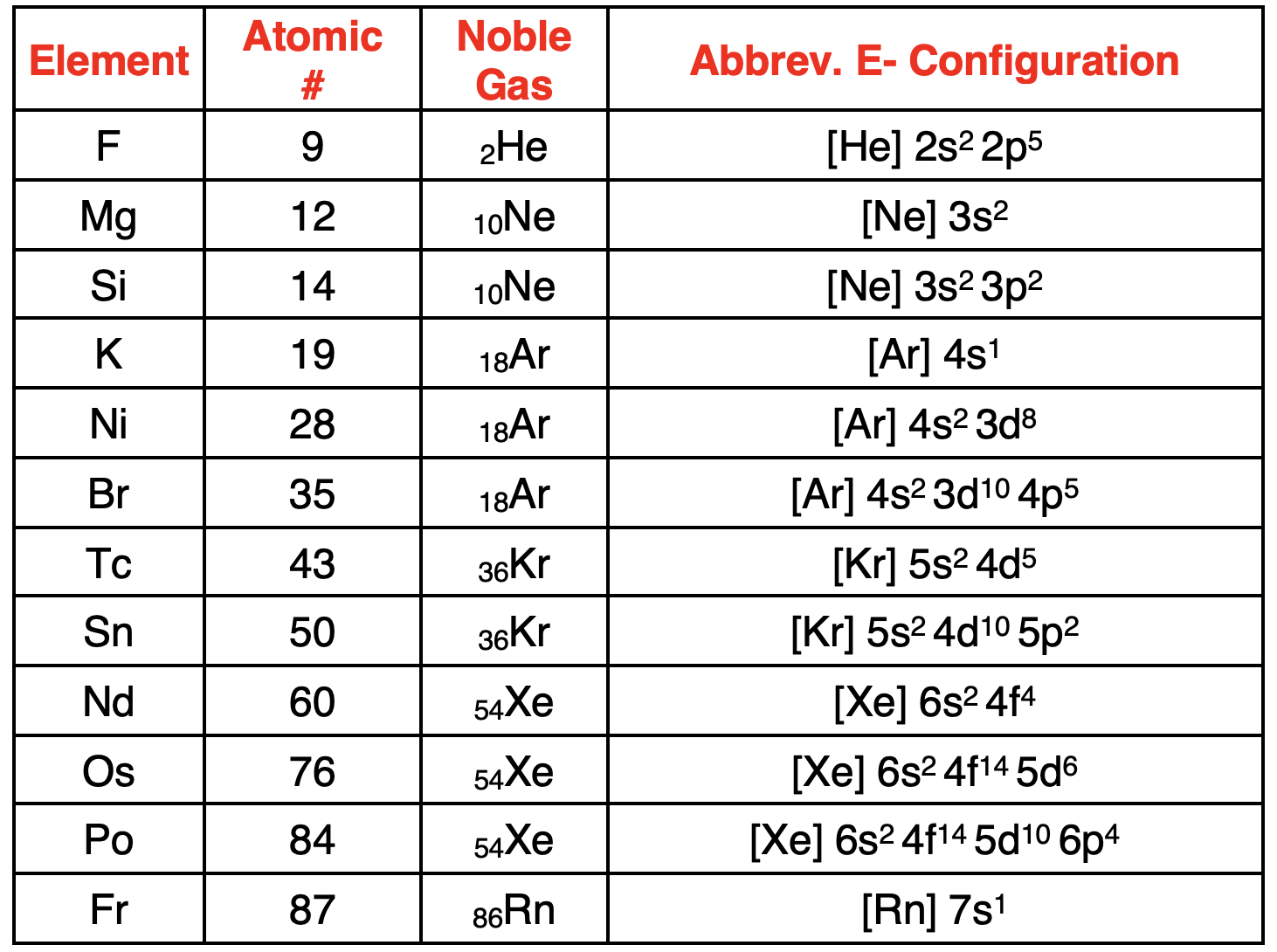
How to Write Electron Configurations for Ions
如何写出离子的电子排布式
Ions have unequal numbers of protons and electrons and are thus charged. An ion can form when an atom gains or loses one or more electrons. Electron configurations can be written for charged ions in the same way they are written for neutral atoms. The first step is to determine the number of electrons in the ion. Look up the atomic number on the periodic table. This indicates the number of protons. Use the atomic number and the charge of the ion to determine the number of electrons. A positively charged ion has fewer electrons than protons. A negatively charged ion has more electrons than protons. The table shows how these principles can be used to determine the number of electrons in an ion.
离子具有不相等的质子数和电子数,因此带有电荷。当原子获得或失去一个或多个电子时,可以形成离子。写出带电离子的电子排布式的方法与写出中性原子的电子排布式的方法相同。第一步是确定离子中的电子数量。在周期表中查找 原子序数。这表示质子的数量。利用原子序数和离子的电荷来确定电子的数量。正电荷离子的电子数少于质子数。负电荷离子的电子数多于质子数。下表展示了如何利用这些原理来确定离子中的电子数量。
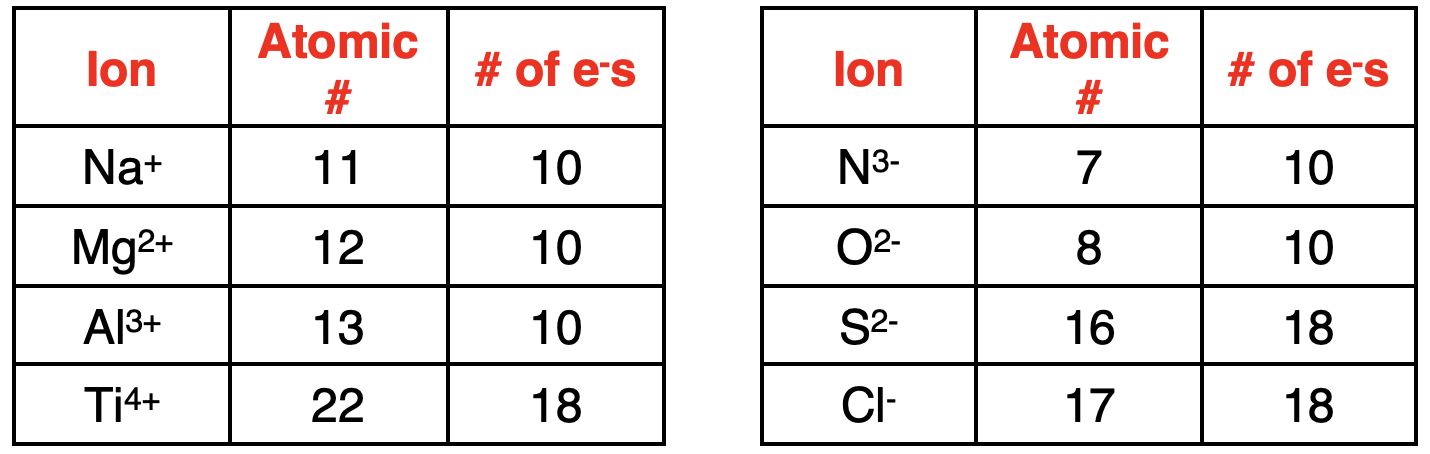
Once the number of electrons is determined, enter the electrons into the orbital box diagram. Then translate the diagram into an electron configuration. Examples are shown below. Note how the electron configuration of a charged ion with 18 electrons is the same as the electron configuration of a neutral atom with 18 electrons. Also note how the main group elements form ions that have the same configuration of electrons as the atoms of noble gas elements.
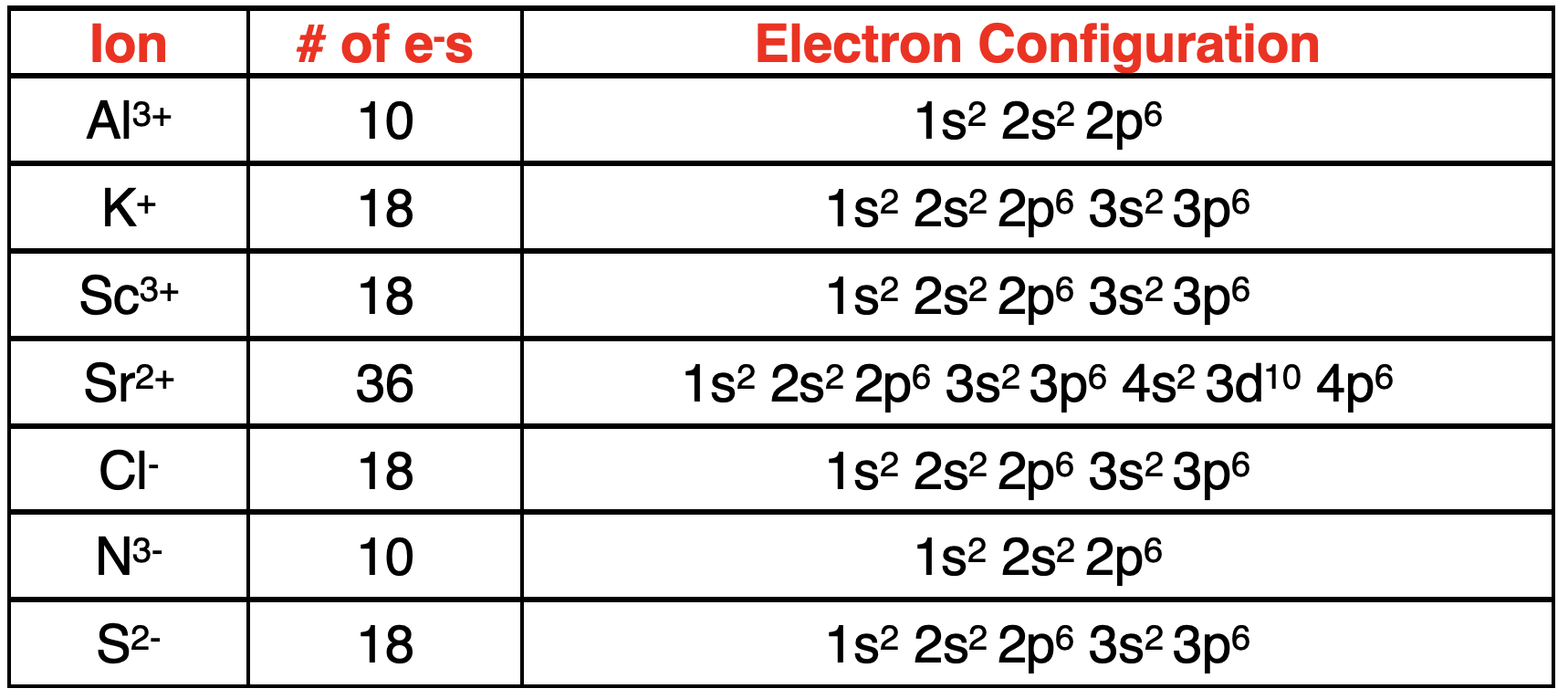
一旦确定了电子的数量,就将电子填入轨道方框图。然后将方框图转化为电子排布式。以下展示了例子。注意,带有 18 个电子的带电离子的电子排布式与带有 18 个电子的中性原子的电子排布式相同。还请注意,主族元素形成的离子的电子排布式与稀有气体元素原子的电子排布式相同。
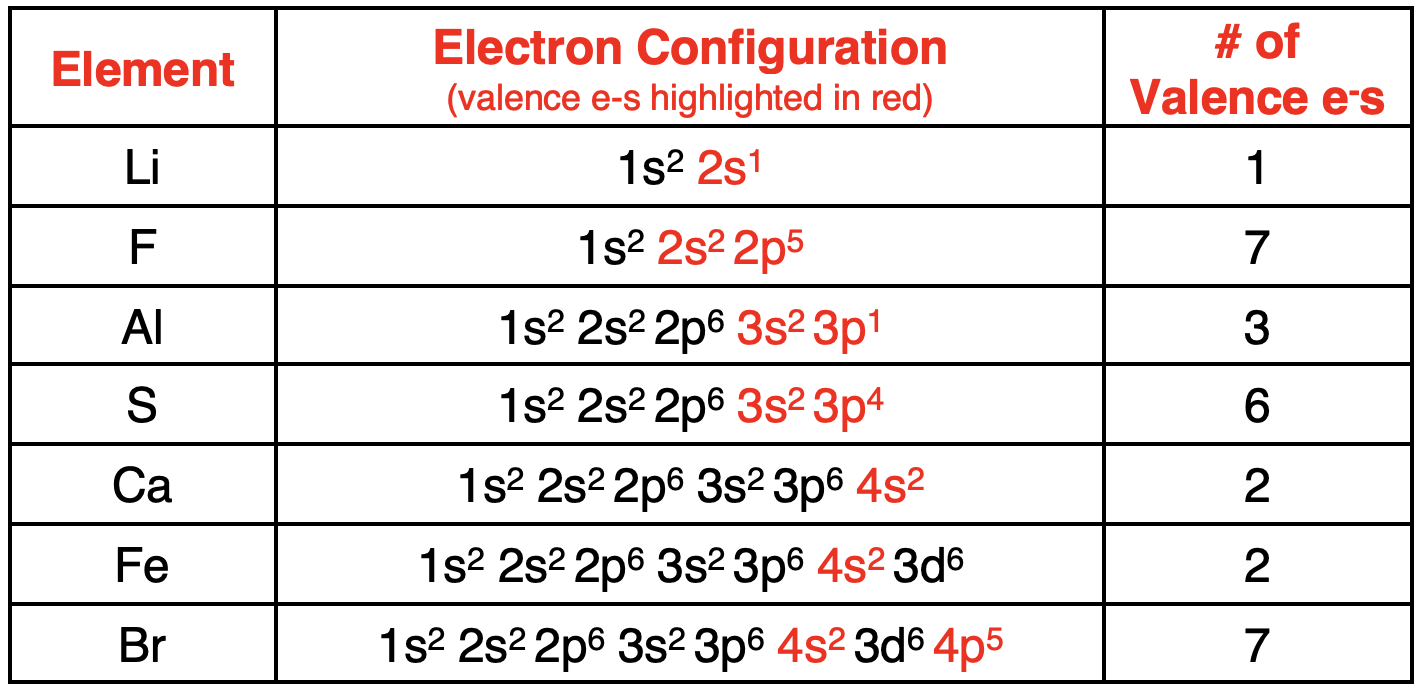
Core Electrons vs. Valence Electrons
内层电子与价电子
The electrons in atoms are located in orbitals. The collection of orbitals at a given energy level (
n
n
n value) form anelectron shell. For instance, the one 2s orbital and the three 2p orbitals form the
n
=
2
n = 2
n=2 electron shell.
原子中的电子位于轨道中。在给定能量层(
n
n
n 值)上的轨道集合形成了一个电子层。例如,一个 2s 轨道和三个 2p 轨道构成了
n
=
2
n = 2
n=2 电子层。
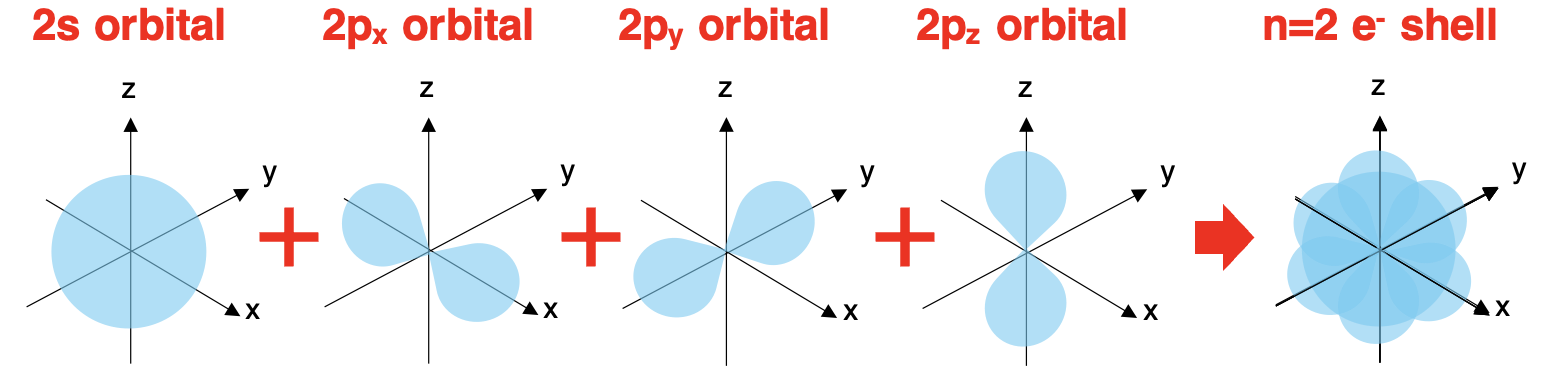
Outer shell electrons, sometimes referred to as valence shell electrons, are s- and p-orbital electrons in the outermost electron shell of atoms.Valence shell electrons are the electrons that are involved in bonding. And because of this, they are the electrons that determine the chemical properties of elements. Whether sodium and oxygen form NaO or NaO₂ or Na₂O or NaO₃ or Na₃O is dependent upon the number of valence shell electrons in the atoms of the two elements.Core electrons are those electrons that are in the inner shells of atoms; they typically do not involve themselves in bonding. The number of valence shell electrons is evident when you inspect an electron configuration.
外层电子,有时也称为价电子层电子,是原子最外层电子层中的 s 轨道和 p 轨道电子。价电子层电子是参与成键的电子。正是因为这一点,它们才是决定元素化学性质的电子。钠和氧是形成 NaO 还是 NaO₂还是 Na₂O 还是 NaO₃还是 Na₃O,取决于这两种元素原子中的价电子层电子数量。内层电子是原子内层中的电子;它们通常不参与成键。从电子排布式中可以清楚地看出价电子层电子的数量。
Note that
d
d
d-orbital electrons are core electrons and are not counted towards the total number of valence electrons. Only
s
s
s- and
p
p
p-orbital electrons are counted towards the total.
请注意,
d
d
d 轨道电子是内层电子,不计入价电子的总数。只有
s
s
s 轨道和
p
p
p 轨道电子计入总数。
Electron Shell Diagrams
电子层示意图
The core and valence shell electrons are often represented by electron shell diagrams. An electron shell diagram shows the electrons layered in shells around a nucleus. Each principal energy level becomes an electron shell and the electrons of that level are represented by an X or a circle on the diagram. The electron shell diagrams for oxygen and magnesium are shown below.
内层电子和价电子通常用电子层示意图表示。电子层示意图展示了围绕原子核分层的电子。每个主能量层成为一个电子层,该层的电子在图中用 X 或圆圈表示。以下展示了氧和镁的电子层示意图。
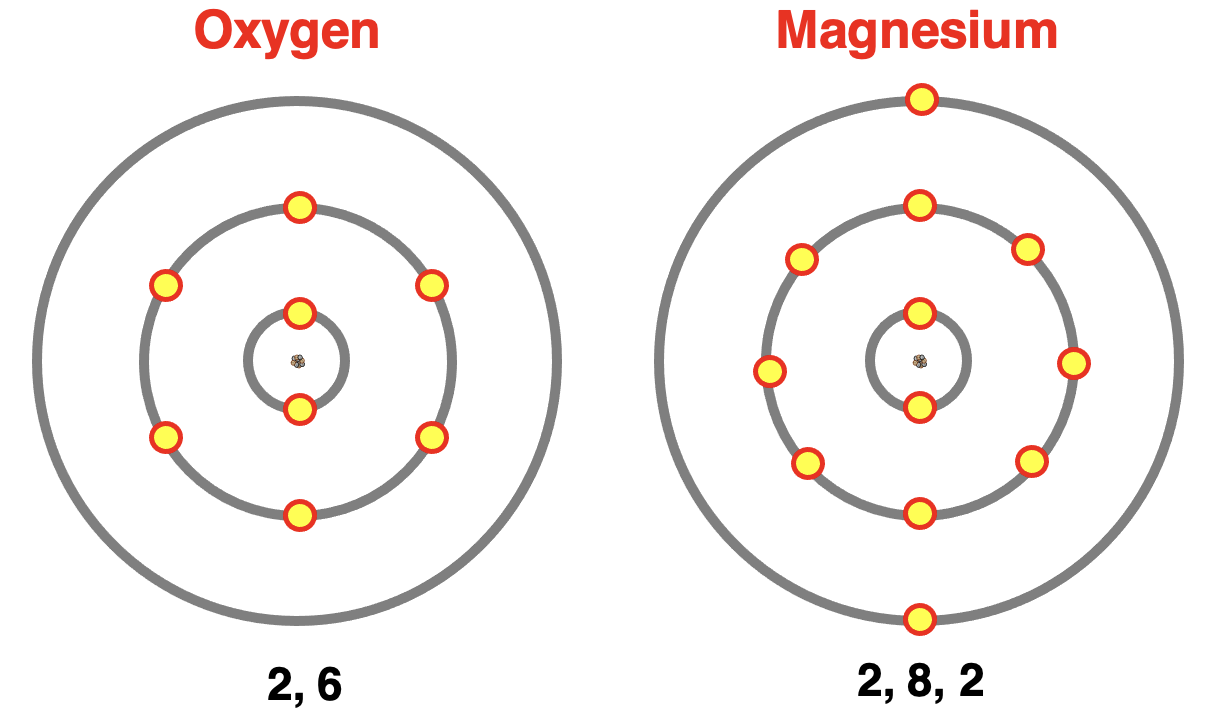
The notation “2, 6” and “2, 8, 2” shown below the diagram is commonly added. The numbers indicate the number of electrons in each shell, beginning with the innermost electron shell. The last number represents the outer shell or valence shell electrons. There are 2 core electrons and 6 valence shell electrons in oxygen. There are a total of 10 core electrons and 2 valence shell electrons in magnesium.
图下方的 “2, 6” 和 “2, 8, 2” 标记通常会被加上。这些数字表示每个电子层中的电子数量,从最内层电子层开始。最后一个数字代表最外层或价电子层电子的数量。氧中有 2 个内层电子和 6 个价电子层电子。镁中共有 10 个内层电子和 2 个价电子层电子。
Electronic Structure of Atoms s,p,d,f Atomic Orbitals
原子的电子结构 s、p、d、f 原子轨道
In today’s post,we will talk about the atomic orbitals.So,first,what is an orbital? In a formal,quantum mechanical definition,orbitals are essentially probability distribution maps for electrons within atoms.
在今天的帖子中,我们将讨论原子轨道。那么,首先,什么是轨道? 从正式的量子力学定义来看,轨道实际上是原子内电子的概率分布图。
In a simpler version,think of orbitals as the place where electrons are located.
用更简单的说法,可以认为轨道是电子所在的位置。
Now,about the types of orbitals and their relative energy levels.There are four types of atomic orbitals – s*,p*,d*,and f*.Each orbital has a characteristic shape shown below:
现在,我们来谈谈轨道的类型及其相对能量级。有四种类型的原子轨道——s*、p*、d* 和 f*。每种轨道都有其特征形状,如下所示:
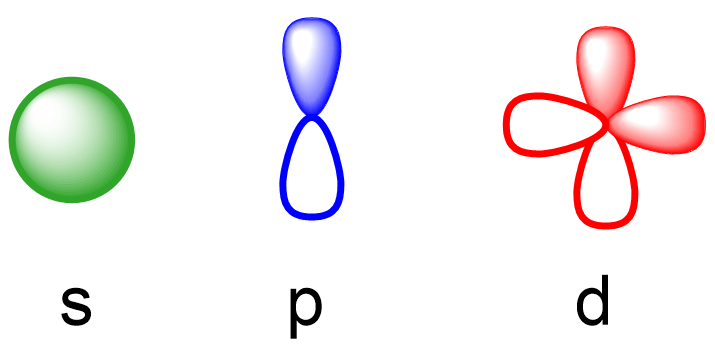
S orbitals have a spherical shape,p* orbitals are dumbbell-shaped,d* orbitals are shaped like a cloverleaf,and f* orbitals are characterized by more complex shapes.You can also look up more detailed images for the shapes and orientation of atomic orbitals in your textbook.
S 轨道呈球形*,p*轨道呈哑铃形*,d*轨道呈三叶草形*,而f*轨道则以更复杂的形状*为特征。你还可以在教科书中查找有关原子轨道形状和方向的更详细图像。
Principal Quantum Number (*n*)
主量子数 (*n*)
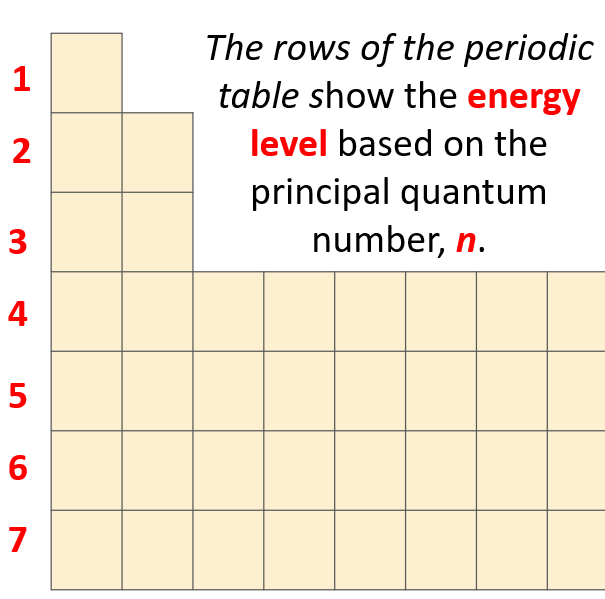
What orbitals a given atom has,and in which ones the electrons are located,depends on the energy level of the atom.Remember,the energy level of the atom is given by the principal quantum number,*n* which can easily be determined based on the period (row) the atom is located in the periodical table.
一个给定原子拥有哪些轨道,以及电子位于哪些轨道中,取决于原子的能量级。记住,原子的能量级由**主量子数,*n***给出,这可以根据原子在周期表中所处的周期(行)轻松确定。
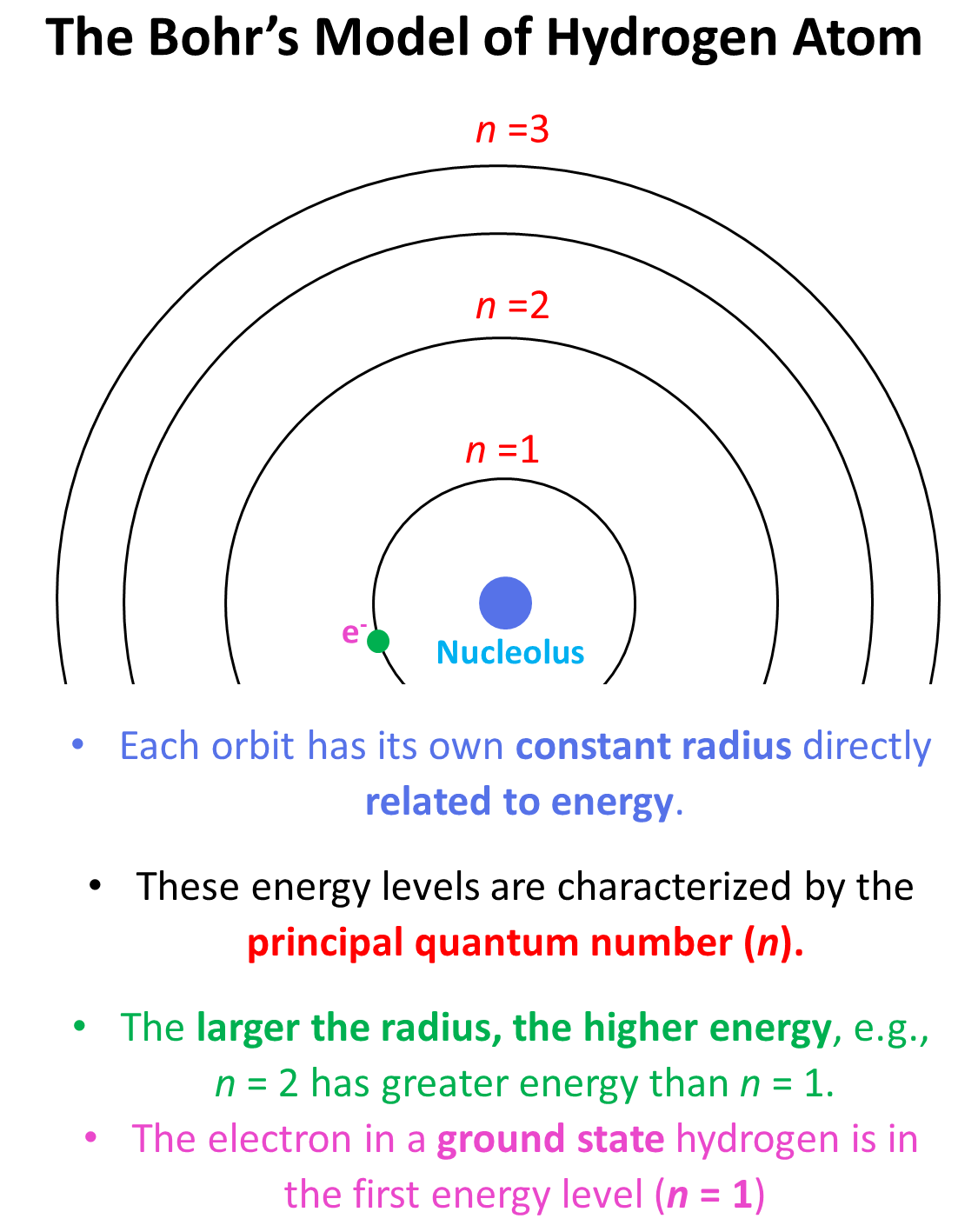
This is what we discussed about the Bohr model of hydrogen atom.There are orbits with fixed radius each associated with discrete energy,and this is described by the principal quantum number n.
这就是我们讨论过的氢原子的玻尔模型。每个轨道都有固定的半径,与离散的能量相关联,这由主量子数 n 描述。
The number of types of orbitals matches the energy level: first energy level has only 1 (s) orbital,the second has two types – s and p,the third has three – s,p,and d,and the fourth level has all four types of orbitals – s,p,d,and f.
轨道类型的数量与能量级相匹配:第一能量级只有 1 个 (s) 轨道,第二能量级有 两种类型——s 和 p,第三能量级有 三种——s、p 和 d,而第四能量级则包含所有 四种类型 的轨道——s、p、d 和 f。
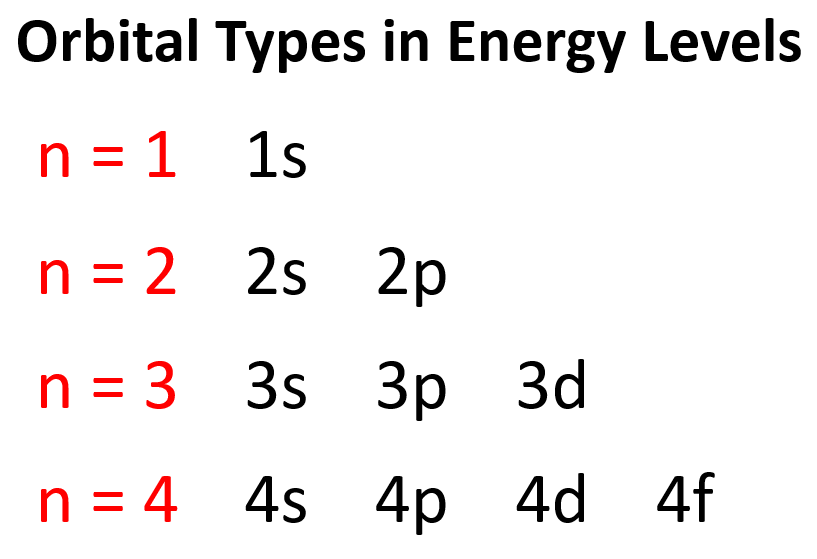
So,far we have talked about the main energy level.However,you should know,aside from the first energy level,each level also has sublevels.These are the types of orbitals – *s*,*p*,*d*,and *f*.
到目前为止,我们已经讨论了主能量级。然而,你应该知道,除了第一能量级外,每个能量级都有亚层。这些就是轨道的类型——*s*、*p*、*d* 和 *f*。
Angular Momentum Quantum Number (*l*)
角动量量子数 (*l*)
The number of sublevels is given by the Angular Momentum Quantum Number – *l*.It takes values of 0,1,… n-1*.For example,for the second energy level,n = 2,and therefore,l = 0,1 , so it can have two values,and therefore,the second energy level has two sublevels – s (l = 0) and p (l = 1).
亚层的数量由角动量量子数——*l*给出。其取值为0、1、……n-1***。例如,对于第二能量级,n = 2,因此,l = 0、1,所以它可以有两个值,因此,第二能量级有两个亚层——s(l = 0)和 p(l = 1)。
Magnetic Quantum Number,*m*l
磁量子数,*m*l
The next quantum number is the Magnetic Quantum Number,ml which shows the number of orbitals in the sublevel.It takes values form –*l* to +*l* including the zero and all the integers.For example,when l = 2,we have d orbitals,and because ml = -2,-1,0,+1,+2,there are 5 orbitals in each sublevel.
下一个量子数是磁量子数,ml,它表示亚层中的轨道数量。其取值范围为 –l 到 +l,包括零和所有整数。例如,当 l = 2 时,我们有 d 轨道,因为 ml = -2、-1、0、+1、+2,所以每个亚层中有 5 个轨道。
The summary of quantum numbers including their meaning and values is given in the diagram below:
量子数的总结,包括它们的含义和值,如下图所示:
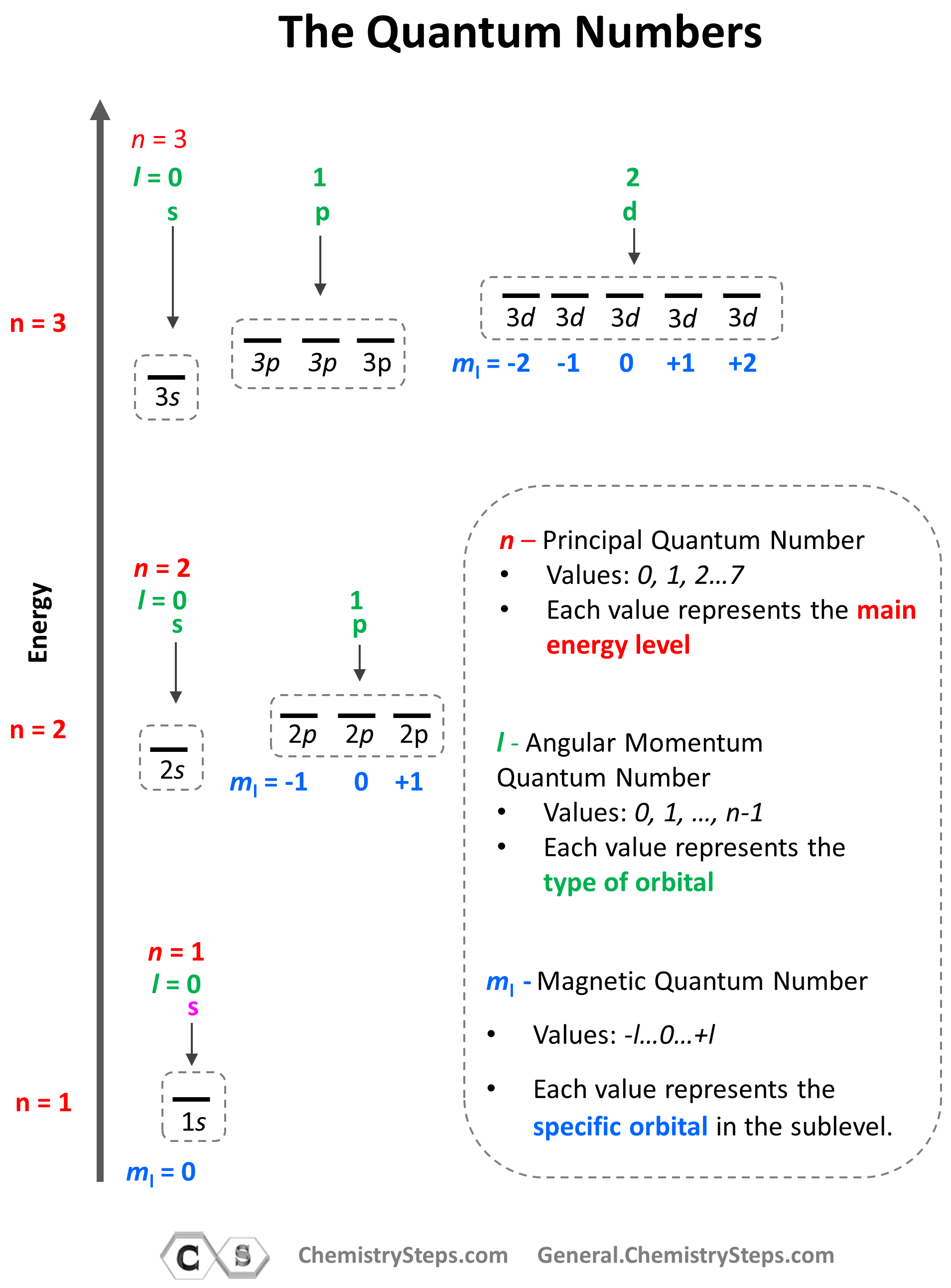
Notice again that within the same principal level,orbitals with a lower value of l have lower energy (E) and therefore,are filled first.So,for a given value of n:
再次注意,在相同的主能级内,l值较低的轨道具有较低的能量 (E),因此会先被填充。因此,对于给定的n值:
E (s orbital) < E ( p orbital) < E (d orbital) < E ( f orbital)
E(s轨道)< E(p轨道)< E(d轨道)< E(f轨道)
Now,a few important things about the orbitals and their electron capacity.First,remember that each orbital,whether it is s,p,d,or f can accommodate two electrons at most.
现在,关于轨道及其电子容量,有几点重要的事情需要注意。首先,记住每个轨道,无论是 s、p、d 还是 f,最多可以容纳两个电子。
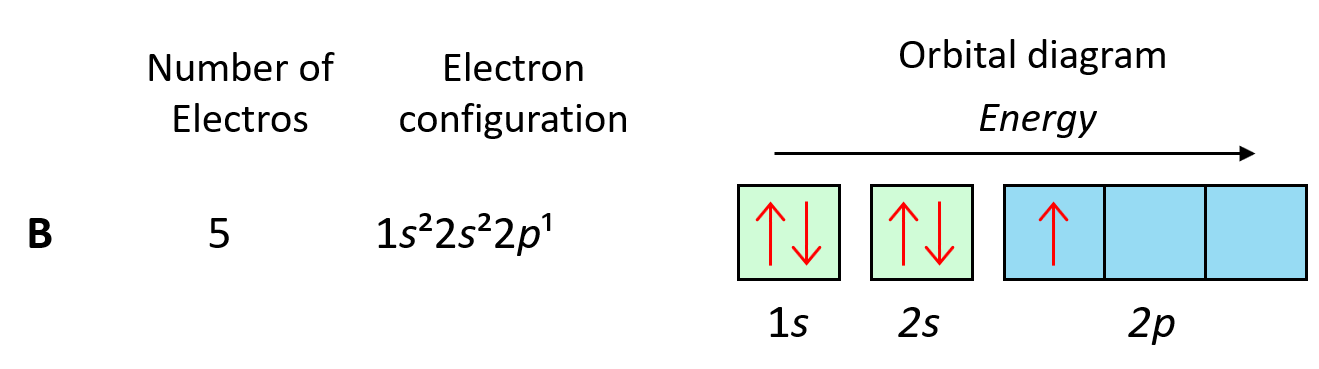
We can see this in orbital diagrams where the orbitals are shown as boxes and electrons as arrows,we never put more than two arrows in the box.For example,boron has two electrons in each s orbital of the first and second levels,and one electron in the p sublevel.
我们可以在轨道图中看到这一点,其中轨道被表示为方框,电子被表示为箭头,我们永远不会在方框中放置超过两个箭头。例如,硼在第一和第二能级的每个 s 轨道中都有两个电子,在 p 亚层中有一个电子。
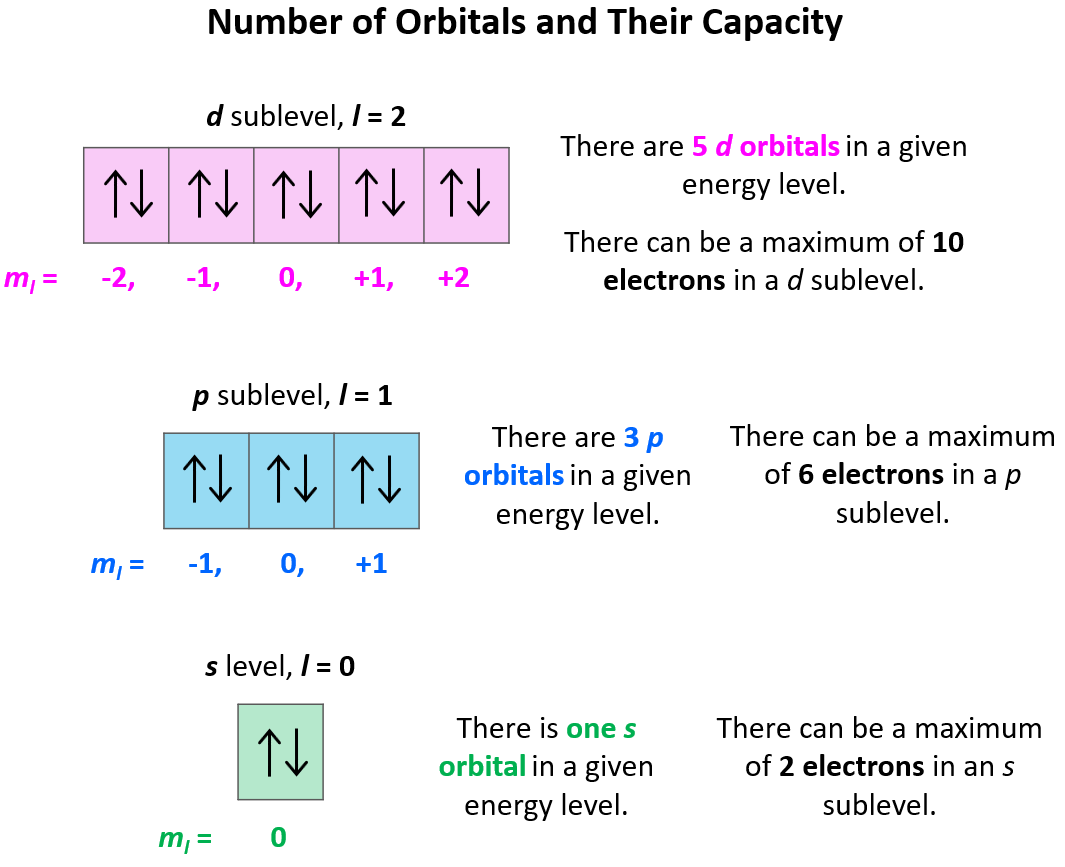
Going back to the quantum numbers,specifically the Magnetic Quantum Number,ml that shows how many types of orbitals we may have in the given sublevel,remember that there can only be 1 *s* orbital in the given energy level,3 *p* orbitals,5 *d* orbitals,and 7 *f* orbitals.And because each orbital can only take a maximum of two electrons,there can only be a maximum of two electrons in any s* sublevel,6* electrons in the p* subshell,10 in the *d*,and 14 in the *f* sublevel.
回到量子数,特别是磁量子数,ml,它显示了在给定亚层中我们可能拥有的轨道类型数量,记住在给定能量级中只能有 1 个 *s* 轨道,3 个 *p* 轨道,5 个*d** 轨道和 7 个 *f* 轨道。而且因为每个轨道最多只能容纳两个电子,所以任何 s 亚层中最多只能有两个电子,p 亚层中最多可以有 6 个电子,d 亚层中可以有 10 个电子,而 f 亚层中可以有14 个电子。
For example,which orbital is indicated by the following set of quantum numbers*: n* = 3,l = 2,ml = 0?
例如,以下量子数集合表示哪个轨道:n = 3,l = 2,ml = 0?
Starting with the principal quantum number,we know that it is an orbital in the 3rd energy level.L = 2,indicates a d orbital,and ml = 0 indicates the middle one of the five d orbitals.
从主量子数开始,我们知道这是一个第 3 能量级的轨道。L = 2 表示这是一个 d 轨道,而 ml = 0 表示这是五个 d 轨道中的中间那个。
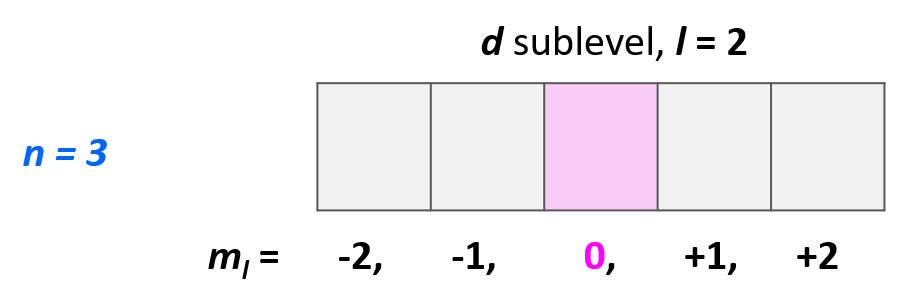
The Electron Spin Quantum Number (*m*s)
电子自旋量子数 (*m*s)
The last quantum number is the Electron Spin Quantum Number (ms) which shows the direction of the electron spin and depending on this may take a value of +1/2,represented by ↑, or -1/2,represented by ↓.Placing the direction of the arrow is important as the electrons in the same orbital may only have opposite spin.
最后一个量子数是电子自旋量子数 (ms),它表示电子自旋的方向,根据此方向可以取值 +1/2,用 ↑ 表示,或 -1/2,用 ↓ 表示。箭头的方向很重要,因为同一轨道中的电子只能具有相反的自旋。
This is the Hund’s rule,which states that electrons will fill all the degenerate orbitals (equal in energy) with parallel spins (both arrows up or down) first before pairing up in one orbital.We can also formulate it as the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons within the same energy sublevel.
这就是 洪特规则,它指出电子会先填满所有简并轨道(能量相等)中的平行自旋(两个箭头都向上或向下),然后才会在同一个轨道中成对。我们也可以将其表述为原子的最低能量构型是在相同的能量亚层中拥有最大数量的未成对电子的那个。
For example, in carbon the second electron in the p sublevel goes to the next (empty) p orbital rather than fitting in with the other electron:
例如,在碳中,p 亚层中的第二个电子会进入下一个(空的)p轨道,而不是与另一个电子配对:
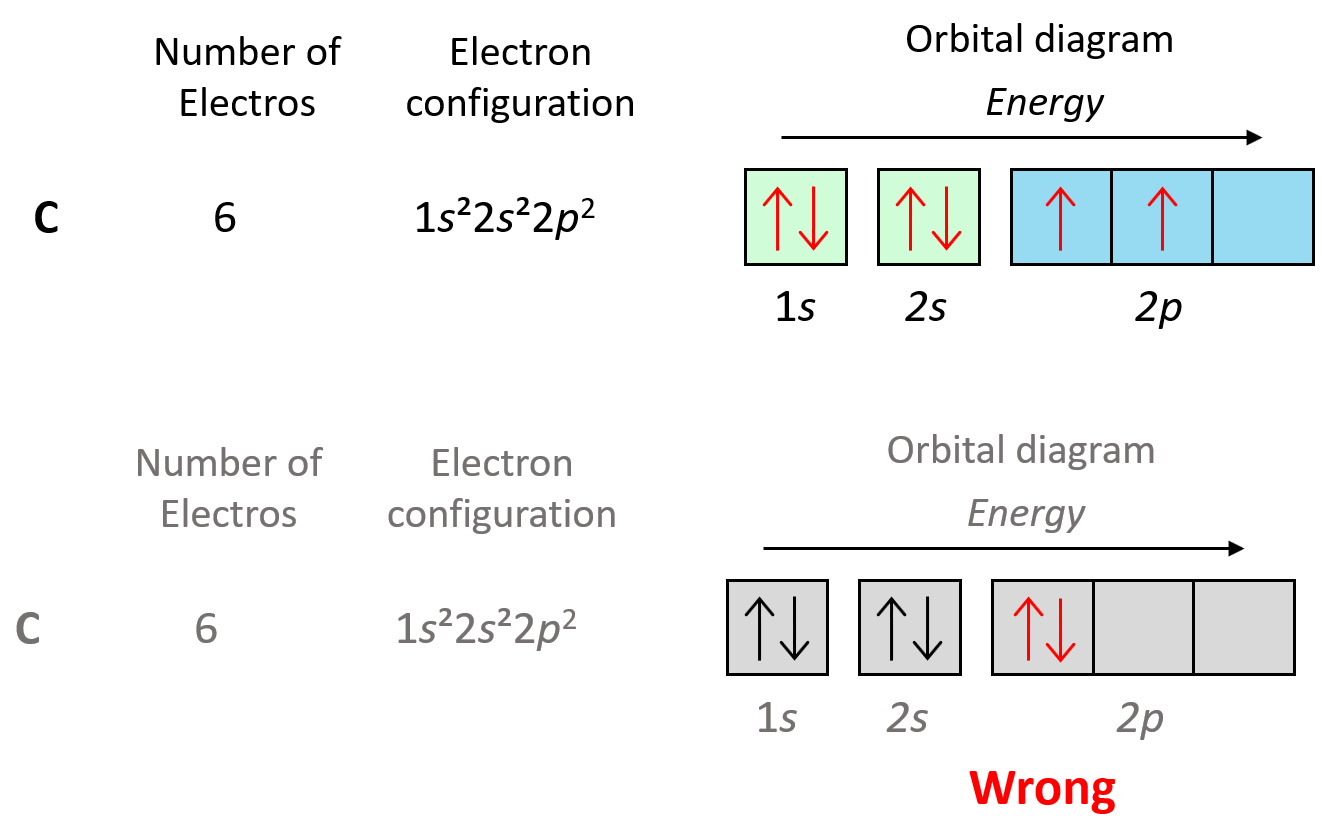
Notice that placing the electron unpaired in the 3s orbital is also incorrect because,it is important to mention,that Hund’s rule applies to electrons in the same energy level.Check this article for more information and exceptions on the Hund’s rule,as well the Aufbau’s and Pauli’s exclusion principles.
请注意,将电子放置在 3s 轨道中且不配对也是错误的,因为需要强调的是,洪特规则适用于同一能量级中的电子。
Rules for the Order of Filling Orbitals with Electrons
电子填充轨道的顺序规则
Electrons are located in orbitals.A single orbital can hold a maximum of two electrons.We will now consider the order in which electrons will fill the orbitals for an atom in the ground state.The number of electrons in a neutral atom will equal the atomic number of the element.We will need to give attention to three principles or rules in order to determine the order by which electrons are placed in the orbitals.
电子位于轨道中。一个轨道最多可以容纳两个电子。我们现在将考虑电子将按顺序填充处于基态的原子的轨道。中性原子中的电子数将等于该元素的原子序数。我们需要关注三个原理或规则,以确定电子放置在轨道中的顺序。
1.Aufbau Principle: electrons first fill the lowest energy orbitals before beginning to fill orbitals with the next highest energy.
奥布夫原理:电子首先填充最低能量的轨道,然后才开始填充能量次高的轨道。
2.Hund’s Rule: For sublevels with more than one orbital (p,d,and f),each orbital gets filled with one electron of the same spin direction before pairing up inside of the same orbital.
洪特规则:对于有多个轨道的亚层(p、d 和 f),每个轨道都会先填充一个自旋方向相同的电子,然后才会在同一个轨道内配对。
3.Pauli Exclusion Principle: When two electrons are in the same orbital,they will have opposite spin direction.
泡利不相容原理:当两个电子处于同一个轨道中时,它们将具有相反的自旋方向。
Orbital Box Diagrams
轨道方框图
The above principles provide guidance regarding the order in which electrons enter the orbitals.We will start applying these rules with some simple cases.The diagrams below are called orbital box diagrams.Each box represents an orbital.They are ordered in order of energy.To save space,they are organized horizontally.Each orbital is labeled.The arrows represent electrons.Arrows pointing in the same direction have the same spin direction; arrows pointing in opposite directions have opposite spin directions.
上述原理为电子进入轨道的顺序提供了指导。我们将从一些简单的情况开始应用这些规则。下面的图表称为轨道方框图。每个方框代表一个轨道。它们按能量顺序排列。为了节省空间,它们被水平排列。每个轨道都有标签。箭头代表电子。指向相同方向的箭头具有相同的自旋方向;指向相反方向的箭头具有相反的自旋方向。
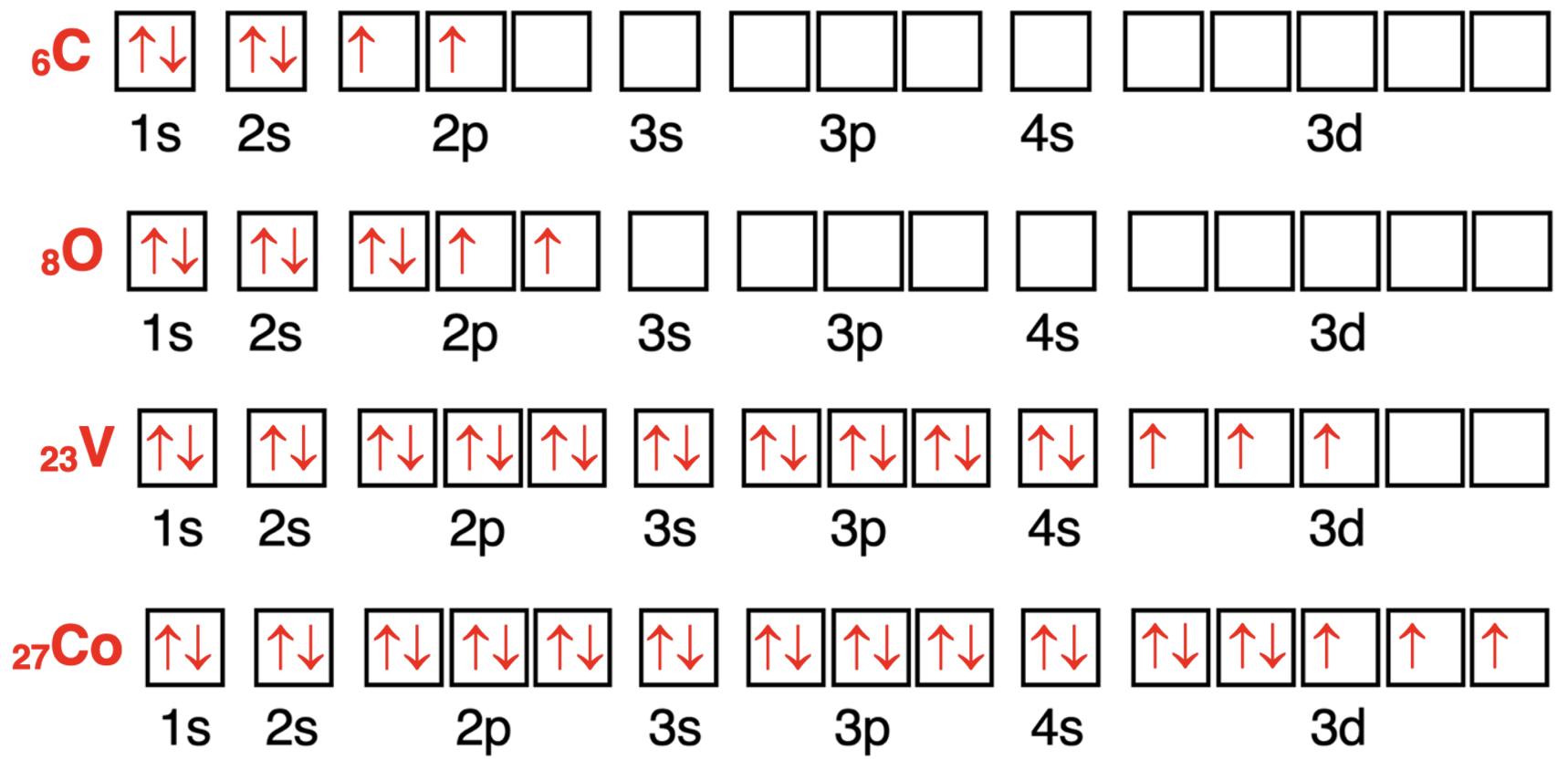
Let’s start with the Lithium atom.Lithium has an atomic number of 3.There are three electrons in the neutral atom.The first two electrons fill the lowest energy 1s orbital (Aufbau Principle).The third electron enters the next highest energy level – the 2s orbital.The orbital box diagram is shown below.
我们先从锂原子开始。锂的原子序数为 3。中性原子中有三个电子。前两个电子填充最低能量的 1s 轨道(奥布夫原理)。第三个电子进入下一个最高能量级——2s 轨道。轨道方框图如下所示。
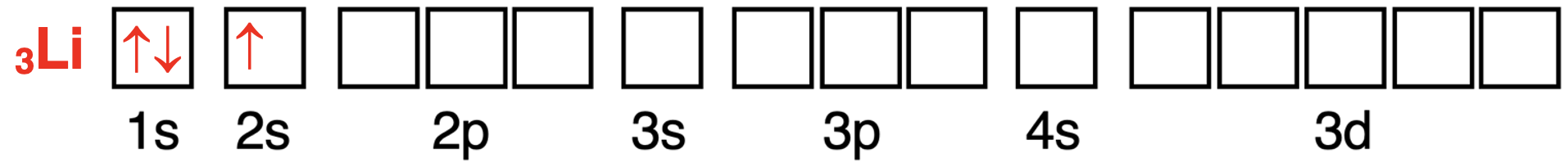
锂原子轨道中电子配置的轨道方框
Now consider the carbon atom with six electrons.The lowest energy s orbitals (1s and 2s) will hold the first four electrons.The fifth electron will go into a 2p orbital.According to Hund’s Rule,the sixth electron will enter a different 2p orbital and have the same spin direction as the fifth electron.The orbital box diagram is shown below.There are two unpaired electrons in the 2p orbitals.
现在考虑有六个电子的碳原子。最低能量的 s 轨道(1s 和 2s)将容纳前四个电子。第五个电子将进入一个 2p 轨道。根据洪特规则,第六个电子将进入另一个 2p 轨道,并且与第五个电子具有相同的自旋方向。轨道方框图如下所示。在 2p 轨道中有两个未成对电子。
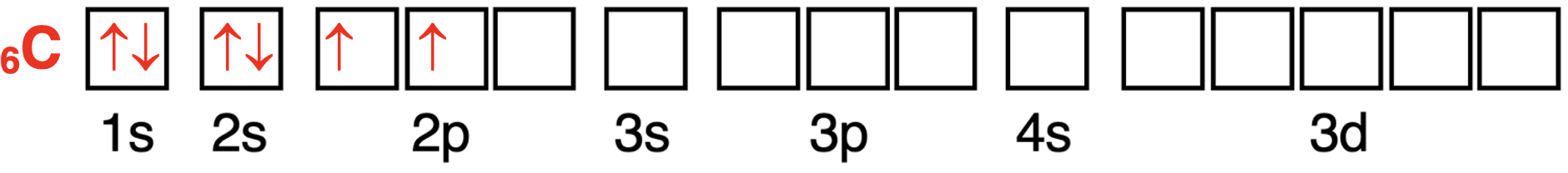
碳原子轨道中电子配置的轨道方框
The oxygen atom has 8 electrons.The first four electrons will enter the 1s and the 2s orbitals.According to Hund’s Rule,the 5th,6th,and 7th electron will enter different 2p orbitals and have the same spin direction.After seven electrons,the 2p orbitals are all half-filled.So,the 8th electron pairs up in a 2p orbital with another electron.According to the Pauli Exclusion Principle,the 8th electron will spin in an opposite direction as the other p orbital electrons.The arrow is drawn in the opposite direction to indicate a different spin direction.There are two unpaired electrons in the 2p orbitals.
氧原子有 8 个电子。前四个电子将进入 1s 和 2s 轨道。根据洪特规则,第 5、6 和 7 个电子将进入不同的 2p 轨道,并且具有相同的自旋方向。在七个电子之后,2p 轨道全部半满。因此,第八个电子将与另一个电子在 2p 轨道中配对。根据泡利不相容原理,第八个电子将与其它 p 轨道电子的自旋方向相反。箭头画在相反方向以表示不同的自旋方向。在 2p 轨道中有两个未成对电子。
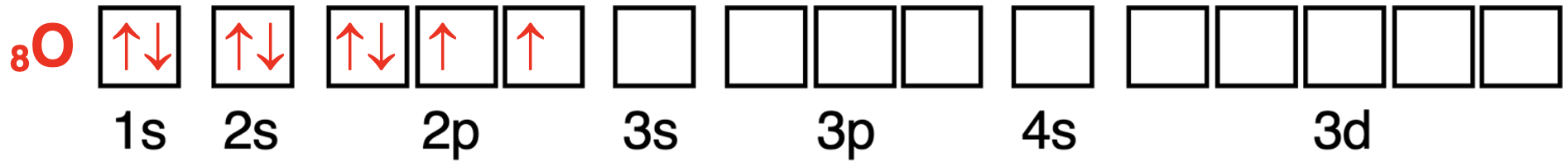
氧原子轨道中电子配置的轨道方框图
The magnesium atom has 12 electrons.The first 10 electrons will fill the lowest energy orbitals.In order,the 1s,the 2s,and the 2p orbitals are filled.The 11th electron enters a 3s orbital.The 12th electron will also enter a 3s orbital and have the opposite spin direction as the 11th electron.There are no unpaired electrons; all electrons are paired up.
镁原子有 12 个电子。前 10 个电子将填充最低能量的轨道。按顺序,1s、2s 和 2p 轨道被填满。第 11 个电子进入一个 3s 轨道。第 12 个电子也将进入一个 3s 轨道,并且与第 11 个电子具有相反的自旋方向。没有未成对电子;所有电子都已配对。
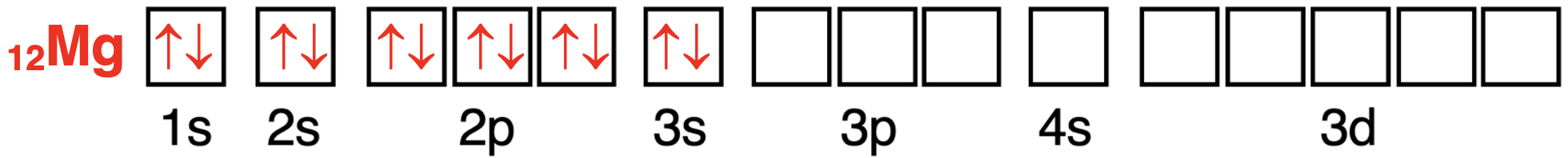
镁原子轨道中电子配置的轨道方框
The first 12 of the 17 electrons in the chlorine atom will fill (in order) the 1s orbital,the 2s orbital,the 2p orbitals,and the 3s orbital.In accordance with Hund’s Rule,the 13th,14th,and 15th electron will enter different 3p orbitals.This will half-fill the 3p orbitals.The 16th and 17th electron will enter two of these 3p orbitals and pair up with the electrons that are already there.This leaves one unpaired electron in the 3p orbitals.
氯原子中的前 12 个电子将按顺序填充 1s 轨道、2s 轨道、2p 轨道和 3s 轨道。根据洪特规则,第 13、14 和 15 个电子将进入不同的 3p 轨道。这将使 3p 轨道半满。第 16 和 17 个电子将进入这两个 3p 轨道,并与已经存在的电子配对。这使得 3p 轨道中留下一个未成对电子。
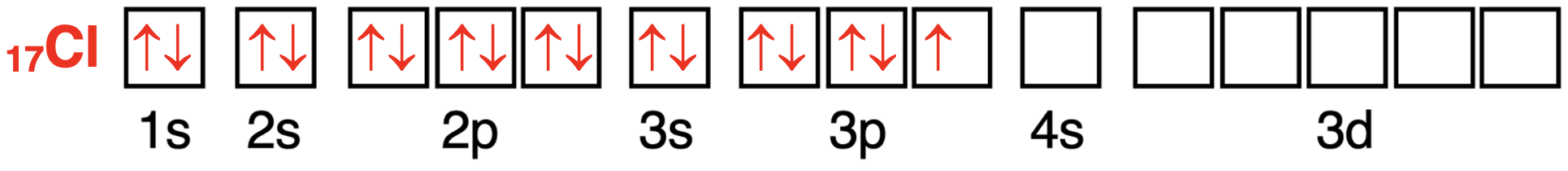
氯原子轨道中电子配置的轨道方框图
The first 18 electrons of potassium’s 19 electrons will enter (in order) the 1s orbital,the 2s orbital,the three 2p orbitals,the 3s orbital,and the three 3p orbitals.Consistent with the Aufbau Principle,the 19th electron will enter the 4s orbital.The 4s orbital is lower in energy than the 3d orbitals.
钾的 19 个电子中的前 18 个将按顺序进入 1s 轨道、2s 轨道、三个 2p 轨道、3s 轨道和三个 3p 轨道。与奥布夫原理一致,第 19 个电子将进入 4s 轨道。4s 轨道的能量低于 3d 轨道。
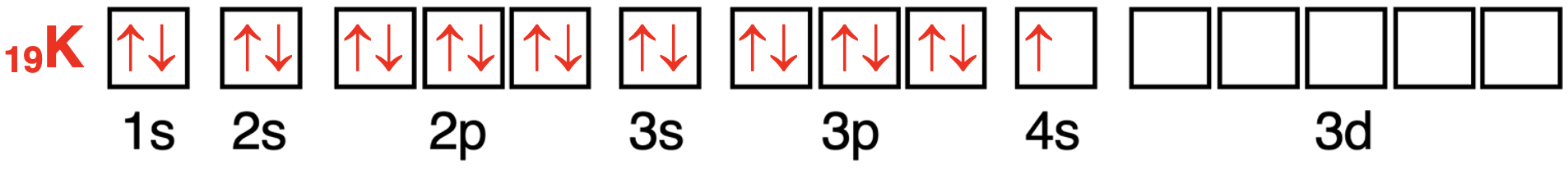
钾原子轨道中电子配置的轨道方框图
Vanadium has 23 electrons.The first 18 electrons will enter (in order) the 1s orbital,the 2s orbital,the three 2p orbitals,the 3s orbital,and the three 3p orbitals.The 19th and 20th electrons will enter the 4s orbital.Finally,the 3d orbitals will begin to fill with the 21st electron.Three of the 3d orbitals will be half-filled with one electron for a total of 23 electrons.There are three unpaired electrons present in the atom.
钒有 23 个电子。前 18 个电子将按顺序进入 1s 轨道、2s 轨道、三个 2p 轨道、3s 轨道和三个 3p 轨道。第 19 和 20 个电子将进入 4s 轨道。最后,3d 轨道将从第 21 个电子开始填充。三个 3d 轨道将各填充一个电子,总共 23 个电子。该原子中有三个未成对电子。
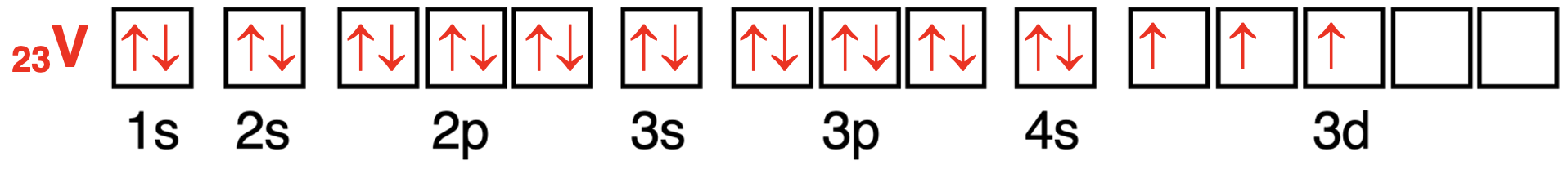
钒原子轨道中电子配置的轨道方框图
Let’s consider a cobalt atom as our final example.It has 27 total electrons.The first 20 electrons will fill in the same way as they did for vanadium.Electrons 21-25 will enter the five different 3d orbitals; none of them will pair up.But the 26th and 27th electron will enter an already half-filled orbital to fill it to capacity.There are three unpaired electrons present in the 3d orbitals and two pairs of paired electrons.
我们以钴原子作为最后一个例子。它总共有 27 个电子。前 20 个电子将像钒一样填充。第 21-25 个电子将进入五个不同的 3d 轨道;它们不会配对。但是第 26 和 27 个电子将进入一个已经半满的轨道,以将其填满。在 3d 轨道中有三个未成对电子和两对配对电子。
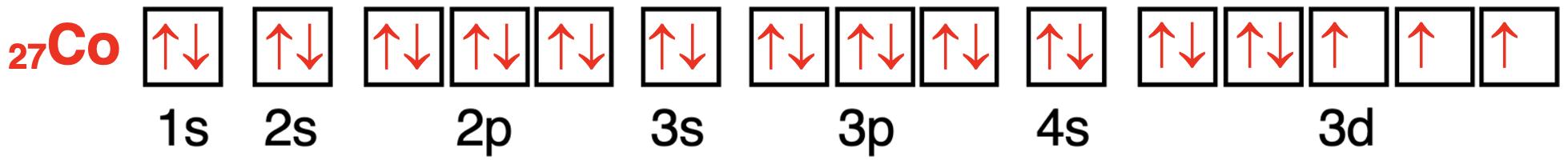
钴原子轨道中电子配置的轨道方框图
The 4 Energy Sublevels Explained: A Simple s,p,d,f Guide
解释 4 个能量亚层:一个简单的 s、p、d、f 指南
Published on 30 August 2025
Forget the simple image of tiny electrons zipping around a nucleus like planets orbiting the sun.While that model is a helpful start,the reality of the atomic world is far more fascinating and organized.Ready to peek inside? Welcome to the true neighborhoods where electrons reside: the atomic sublevels.
忘掉那种简单的小电子像行星绕太阳运行一样绕着原子核快速移动的图像吧。虽然那个模型是一个很好的开始,但原子世界的现实要复杂得多,也更有条理。准备好往里瞧瞧了吗?欢迎来到电子真正居住的地方:原子亚层。
In this guide,we will crack the code of atomic structure by demystifying the four fundamental energy sublevels—s,p,d,and f.Understanding these distinct regions is the key to mastering electron configuration and unlocking the secrets of the Periodic Table.We’ll discover how their unique shapes,defined by a concept from quantum mechanics called the Azimuthal Quantum Number,dictate the very foundation of chemistry.Let’s begin our journey into the quantum realm!
在这份指南中,我们将通过解开四个基本能量亚层——s、p、d 和 f的神秘面纱,来破解原子结构的密码。理解这些不同的区域是掌握电子构型和揭开周期表秘密的关键。我们将发现,它们独特的形状由量子力学中的一个概念——方位量子数定义,它们决定了化学的基础。让我们开始我们的量子领域之旅吧!

Image taken from the YouTube channel Joedelyn Cruz ,from the video titled Energy Levels,Sublevels,and Orbitals .
Moving beyond the simplistic image of electrons as tiny planets orbiting a central sun,the true landscape within an atom is far more nuanced and fascinating.
超越那种将电子视为围绕中心太阳运行的小行星的简单图像,原子内部的真实景观要复杂得多,也更有趣。
Beyond Simple Orbits: Mapping the Electron’s Quantum Neighborhoods
超越简单轨道:绘制电子的量子邻域
Welcome! To truly demystify the world inside an atom,we must venture beyond the basic model of electrons orbiting a nucleus in ill-defined paths.The reality is that electrons occupy specific regions of space,guided by the principles of quantum mechanics.Understanding these regions is like cracking the atom’s secret code,revealing how it interacts with the world around it.
欢迎!要真正揭开原子内部世界的神秘面纱,我们必须超越那种电子在不明确路径上绕原子核运行的基本模型。现实是,电子占据了特定的空间区域,受到量子力学原理的引导。理解这些区域就像破解原子的秘密代码,揭示它如何与周围的世界相互作用。
The Electron’s True Home: Energy Levels and Sublevels
电子的真正家园:能量级和亚层
Instead of random orbits,electrons reside in distinct areas characterized by their energy.We introduce two core concepts here:
与其说是随机轨道,不如说电子居住在由其能量特征的特定区域。这里我们引入两个核心概念:
-
Energy Levels (or Shells): Imagine these as floors in a multi-story building around the nucleus.Each floor represents a principal energy level,and electrons on higher floors possess more energy.These are often denoted by the principal quantum number (n = 1,2,3,etc.).
能量级(或壳层):想象这些是围绕原子核的多层建筑中的楼层。每一层代表一个主能量级,位于更高楼层的电子具有更多的能量。这些通常用主量子数(n = 1、2、3 等)表示。 -
Atomic Sublevels (or Subshells): Within each energy level (or floor),there are further subdivisions – like different types of apartments on that floor.These are the specific “neighborhoods” where electrons are most likely to be found.They are designated by the letters s,p,d,and f.Each of these sublevels has a unique shape and can hold a specific number of electrons.
原子亚层(或亚壳层):在每个能量级(或楼层)内,还有进一步的细分——就像那层楼上有不同类型的公寓。这些是电子最有可能被找到的特定“街区”。它们用字母s、p、d 和 f表示。这些亚层每个都有独特的形状,并且可以容纳特定数量的电子。
Why Sublevels Matter: Your Key to Chemistry
为什么亚层很重要:化学的关键
Understanding these atomic sublevels is absolutely fundamental to mastering two cornerstone concepts in chemistry:
理解这些原子亚层是掌握化学中两个基石概念的绝对基础:
-
Electron Configuration: This is essentially the “address” for every electron in an atom.Knowing which sublevels exist and how many electrons they can hold allows us to precisely map where all the electrons in an atom are,which dictates its chemical behavior.
电子构型:这实际上是原子中每个电子的“地址”。知道哪些亚层存在以及它们可以容纳多少电子,使我们能够精确地绘制出原子中所有电子的位置,这决定了它的化学行为。 -
Interpreting the Periodic Table: The structure of the Periodic Table,from the way elements are grouped into blocks to their recurring properties,is a direct reflection of how electrons fill these s,p,d,and f sublevels.It’s the ultimate roadmap to understanding elemental relationships.
解读周期表:周期表的结构,从元素被分组为块的方式到它们的周期性属性,是电子填充这些 s、p、d 和 f 亚层的直接反映。它是理解元素关系的终极路线图。
By grasping these concepts,you’ll gain profound insight into why elements react the way they do,why some are stable,and why others are highly reactive.It’s the key to predicting chemical bonds and the properties of materials.
通过掌握这些概念,你将深刻洞察元素为何会以这种方式发生反应,为什么有些元素稳定,而另一些元素高度活跃。这是预测化学键和材料性质的关键。
The Quantum Clues: Azimuthal Quantum Number (l)
量子线索:方位量子数(l)
So,what defines these specific sublevels and their distinct shapes? The answer lies in the world of Quantum Numbers.While there are several quantum numbers,the particular one that dictates the type and shape of an atomic sublevel is the Azimuthal Quantum Number (l),also sometimes called the orbital angular momentum quantum number.Each value of ‘l’ corresponds to a specific sublevel type:
那么,是什么定义了这些特定的亚层及其独特的形状呢?答案在于量子数的世界。尽管有好几个量子数,但决定原子亚层类型和形状的特定量子数是方位量子数(l),有时也称为轨道角动量量子数。每个 ‘l’ 值对应一个特定的亚层类型:
- l = 0 corresponds to the s sublevel
l = 0对应于s亚层 - l = 1 corresponds to the p sublevel
l = 1对应于p亚层 - l = 2 corresponds to the d sublevel
l = 2对应于d亚层 - l = 3 corresponds to the f sublevel
l = 3对应于f亚层
These quantum numbers are like the architectural blueprints that define the structure of the electron’s home.
这些量子数就像定义电子家园结构的建筑设计蓝图。
A Quick Guide to Atomic Sublevels
原子亚层快速指南
To summarize,here’s a table outlining the key characteristics of the s,p,d,and f sublevels:
总结一下,下面是一个表格,概述了 s、p、d 和 f 亚层的关键特征:
| Sublevel | Azimuthal Quantum Number (l) | Number of Atomic Orbitals | Maximum Electron Capacity |
|---|---|---|---|
| s | 0 | 1 | 2 |
| p | 1 | 3 | 6 |
| d | 2 | 5 | 10 |
| f | 3 | 7 | 14 |
Each “atomic orbital” within a sublevel represents a specific region of space that can hold up to two electrons.As you can see,the higher the ‘l’ value,the more orbitals a sublevel contains,and thus the greater its electron capacity.
亚层中的每个“原子轨道”代表一个特定的空间区域,可以容纳多达两个电子。正如你所看到的,‘l’ 值越高,亚层包含的轨道越多,因此其电子容量越大。
Now that we’ve introduced the concept of these quantum neighborhoods,let’s dive into the simplest and most fundamental of them: the s-sublevel.
现在我们已经介绍了这些量子邻域的概念,让我们深入探讨其中最简单、最基本的:s-亚层。
Having grasped the foundational concept of energy sublevels,we’re now ready to zoom in and explore the specific “neighborhoods” where electrons reside,starting with the simplest.
掌握了能量亚层的基础概念后,我们现在准备深入探索电子居住的具体“街区”,从最简单的开始。
Your First Orbital Adventure: Unveiling the Simple, Spherical s-Sublevel
你的首次轨道探索之旅:揭秘简单的球形 s-亚层
Welcome to your initial deep dive into the specific arrangements of electrons within an atom! The s-sublevel is our starting point, and fittingly so, as it represents the lowest energy state for electrons within any given principal Energy Level. Think of it as the ground-floor apartment in a multi-story building – it’s the easiest and most stable place for an electron to occupy.
欢迎开启首次深入探索原子内电子具体排布的旅程!s-亚层是我们的起点,这一选择十分恰当,因为它代表了电子在任意给定主能量级中的最低能量状态。可以将其比作多层建筑中的一楼公寓——这里是电子最易占据且最稳定的位置。
The Atom’s Spherical Sanctuary: Understanding s-Orbital Shape
原子的球形“庇护所”:解读 s-轨道的形状
One of the most defining characteristics of the s-sublevel is the shape of its Atomic Orbital. Imagine a perfectly symmetrical ball, or a sphere, surrounding the atom’s nucleus. That is precisely what an s-orbital looks like.
s-亚层最显著的特征之一便是其原子轨道的形状。试想一个完美对称的球(或球体)围绕在原子核周围——这正是s-轨道的形态。
-
A Perfect Sphere: This spherical shape means that electrons in an s-orbital have an equal probability of being found in any direction around the nucleus, provided they are at a specific distance. It is perfectly symmetrical, with the nucleus at its exact center.
完美的球体:这种球形结构意味着,只要处于特定距离,s-轨道中的电子在原子核周围任意方向出现的概率均相等。s-轨道具有完美的对称性,原子核恰好位于其中心。 -
Size Matters, Shape Doesn’t: While all s-orbitals are spherical, they vary in size. For instance, the 1s orbital (located in the first Energy Level) is smaller and held more tightly to the nucleus than the 2s orbital (found in the second Energy Level). As you move to higher Energy Levels (such as 3s, 4s, etc.), s-orbitals grow progressively larger – yet they all retain that distinct spherical geometry.
尺寸有别,形状不变:尽管所有 s-轨道均为球形,但其尺寸并不相同。例如,(位于第一个能量级的)1s 轨道比(位于第二个能量级的)2s 轨道更小,且与原子核结合得更紧密。当进入更高的能量级(如 3s、4s 等)时,s-轨道会逐渐变大,但始终保持独特的球形几何结构。
The s-Sublevel’s Exclusive Guest List: Electron Capacity
s-亚层的“专属客容量”:电子容纳限度
Despite its importance as a starting point, the s-sublevel has a very limited capacity for hosting electrons.
尽管 s-亚层是重要的起点,但其容纳电子的能力却十分有限。
-
Just One Orbital: Each s-sublevel contains only a single Atomic Orbital. This is a key difference between the s-sublevel and other sublevels we will encounter later.
仅含一个轨道:每个s-亚层仅包含一个原子轨道——这是 s-亚层与我们后续将接触到的其他亚层的关键区别。 -
Two Electron Limit: In accordance with the fundamental Pauli Exclusion Principle, this single s-orbital can hold a maximum of two electrons. These two electrons must have opposite spins, ensuring no two electrons in an atom share the exact same set of quantum numbers. A simple analogy: it’s like having two passengers in a single-seat car, as long as one faces forward and the other backward (this is a simplified way to understand spin!).
最多容纳两个电子:根据基本的泡利不相容原理,这个单一的 s-轨道最多可容纳两个电子。这两个电子必须具有相反的自旋方向,以确保原子中没有两个电子拥有完全相同的量子数组合。可以简单类比为:单人座汽车里坐了两名乘客,但前提是一人面朝前方、一人面朝后方(这是对“自旋”的简化理解!)。
Putting it into Practice: s-Sublevel Electron Examples
实践应用:s-亚层电子排布实例
To make this concept more concrete, let’s examine how the s-sublevel is filled in some of the simplest atoms:
为让这一概念更易理解,我们来看看最简单的几种原子中 s-亚层的电子填充情况:
-
Hydrogen (H): As the first element on the periodic table, hydrogen has only one electron. This electron naturally occupies the lowest available energy state – the 1s orbital. Its electron configuration is therefore written as 1s¹ (meaning one electron in the 1s orbital).
氢(H):作为周期表中的第一个元素,氢仅有一个电子。该电子会自然占据能量最低的可用轨道——1s 轨道。因此,氢的电子构型表示为1s¹(即 1s 轨道中有 1 个电子)。 -
Helium (He): With two electrons, helium completely fills the 1s orbital. Its electron configuration is 1s² (meaning two electrons, with opposite spins, in the 1s orbital). This full s-sublevel makes helium extremely stable and unreactive.
氦(He):氦拥有两个电子,恰好填满 1s 轨道。其电子构型为1s²(即 1s 轨道中有两个自旋方向相反的电子)。完全充满的 s-亚层使氦具有极高的稳定性,且不与其他物质发生反应。
Understanding the simple, spherical s-sublevel lays a crucial foundation. However, the atomic world soon presents us with more complex electron arrangements, featuring intriguing shapes.
理解简单的球形 s-亚层是掌握原子结构的关键基础,但原子世界中还存在更多结构复杂、形态奇特的电子排布方式。
Now that you’ve mastered the simple, spherical s-sublevel – where electrons reside in a neat, uniform “sphere” – you’re ready to explore a slightly more complex, yet equally fascinating, “neighborhood” of electrons.
既然你已掌握了简单的球形 s-亚层(电子在其中整齐、均匀地分布在“球体”中),接下来就可以探索一个稍复杂但同样有趣的电子“区域”了。
Unpacking the Peculiar p-Sublevel: Where Electrons Get Directional Homes
解析特殊的 p-亚层:电子的“定向居所”
As you move beyond the first energy level (n = 1), the world of electron orbitals expands, bringing new shapes and possibilities. The p-sublevel makes its formal appearance starting from the second Energy Level (n = 2), and its unique structure has a profound impact on how atoms interact. Unlike the s-sublevel – a single spherical shell – the p-sublevel introduces greater complexity and directionality.
当探索超越第一个能量级(n = 1)时,电子轨道的世界随之扩展,呈现出新的形态与可能性。p-亚层从第二个能量级(n = 2)开始正式出现,其独特结构对原子间的相互作用有着深远影响。与 s-亚层(单一球形壳层)不同,p-亚层的结构更复杂,且具有方向性。
The Distinctive Shapes of p-Orbitals
p-轨道的独特形态
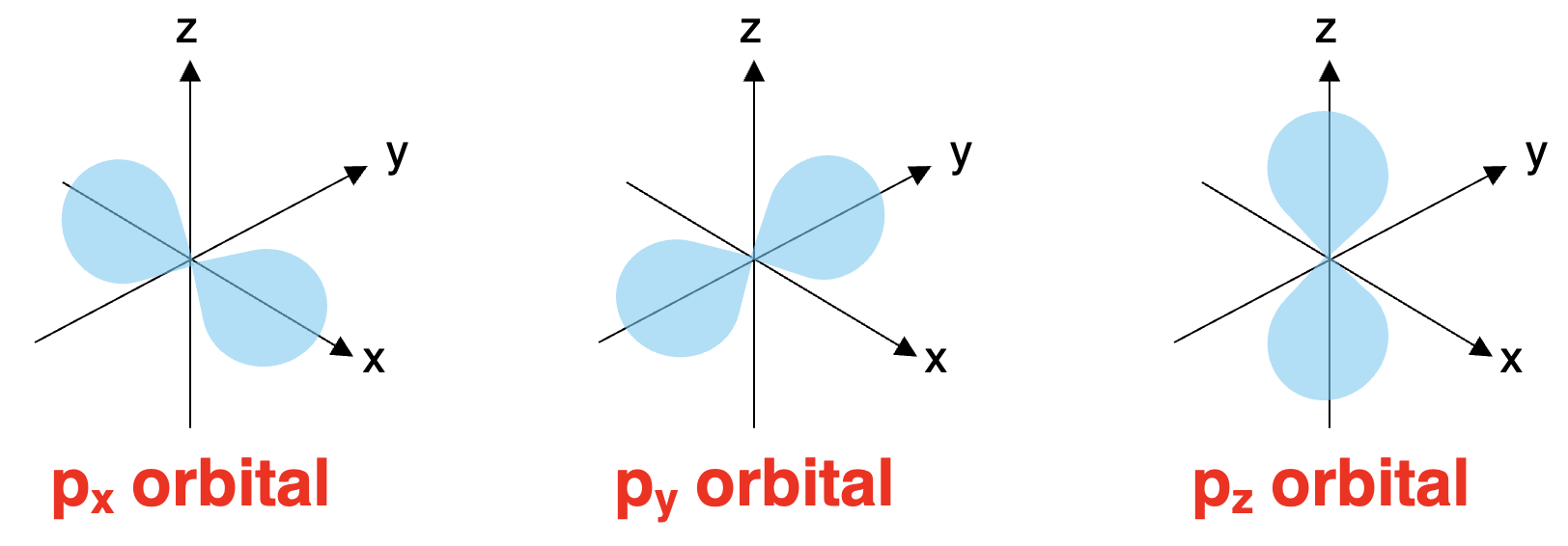
Forget the simple sphere – p-orbitals are often described as having a dumbbell shape, or more colloquially, a peanut shape. But here’s the interesting part: the p-sublevel isn’t just one “dumbbell” – it consists of three distinct dumbbell-shaped Atomic Orbitals. Imagine three dumbbells, each perfectly aligned along one of the three spatial axes: x, y, and z.
忘掉简单的球体吧——p-轨道的形状常被描述为哑铃形,通俗来说也像花生形。但有趣的是:p-亚层并非只有一个“哑铃”,而是由三个不同的哑铃形原子轨道组成。可以想象三个哑铃,分别沿 x、y、z 三个空间轴完美排列。
-
px orbital: This dumbbell-shaped orbital is oriented along the x-axis.
px 轨道:该哑铃形轨道沿x 轴方向排布。 -
py orbital: This one lies along the y-axis.
py 轨道:该轨道沿y 轴方向排布。 -
pz orbital: This orbital extends along the z-axis.
pz 轨道:该轨道沿z 轴方向延伸。
These three orbitals are identical in shape and energy (they are degenerate), but their different spatial orientations mean electrons in these orbitals have a directional preference – a key difference from the non-directional s-orbital.
这三个轨道的形状和能量完全相同(即“简并轨道”),但空间取向不同,这使得占据这些轨道的电子具有方向性偏好——这与无方向性的 s-轨道 形成了关键区别。
Electron Capacity: How Many Can Fit?
电子容量:p-亚层能容纳多少电子?
Since the p-sublevel consists of three separate atomic orbitals (px, py, and pz), and each orbital can hold a maximum of two electrons, the entire p-sublevel has a total maximum electron capacity of six electrons (3 orbitals × 2 electrons per orbital = 6 electrons). This means an atom can place up to six electrons in the p-orbitals of a given energy level.
由于 p-亚层 包含三个独立的原子轨道(px、py、pz),且每个轨道最多容纳两个电子,因此整个p-亚层的最大电子容量为 6 个电子(3 个轨道 × 每个轨道 2 个电子 = 6 个电子)。这意味着,在给定的能量级中,一个原子最多可在其 p-轨道中填充 6 个电子。
Hund’s Rule: The “One Electron Per Seat” Policy
洪特规则:“一轨道一电子”原则
When electrons start filling these three equally energetic p-orbitals, they follow a key principle known as Hund’s Rule. This rule states: electrons will occupy each of the three p-orbitals singly before any orbital gains a second electron. Think of it this way: if you board a bus with several empty double seats, you’d likely sit alone in an empty seat before sitting next to someone else – electrons do the same to minimize repulsion between them.
当电子开始填充这三个能量相等的 p-轨道 时,会遵循一项重要原则——洪特规则。该规则指出:在任意一个 p-轨道获得第二个电子之前,电子会先单独占据三个 p-轨道中的每一个。可以这样理解:如果你登上一辆有多个空双人座的公交车,你可能会先独自坐在空座位上,而非立刻与他人邻座——电子也会如此,以最大限度减少彼此间的排斥力。
Here’s how this works for p-orbitals:
以下是 p-轨道 中电子填充的具体过程:
-
The first electron occupies one
p-orbital(e.g., px).
第一个电子占据一个 p-轨道(如 px 轨道)。 -
The second electron moves into a different
p-orbital(e.g., py).
第二个电子进入另一个 p-轨道(如 py 轨道)。 -
The third electron fills the last remaining empty
p-orbital(e.g., pz).
第三个电子填充最后一个空的 p-轨道(如 pz 轨道)。 -
Only after each
p-orbitalhas one electron do subsequent electrons begin to pair up: the fourth electron enters px, the fifth enters py, and the sixth enters pz.
只有当每个 p-轨道都拥有一个电子后,后续电子才开始配对:第四个电子进入 px 轨道,第五个进入 py 轨道,第六个进入 pz 轨道。
Seeing Hund’s Rule in Action: The Case of Carbon
洪特规则的实例:以碳元素为例
Let’s use Carbon to illustrate Hund’s Rule. Carbon has an atomic number of 6, meaning it has 6 electrons. Its electron configuration is 1s²2s²2p².
我们以碳元素为例解读洪特规则。碳的原子序数为 6,即拥有 6 个电子,其电子构型为1s²2s²2p²。
-
1s²: Two electrons fill the spherical
1s orbital.
1s²:两个电子填满球形的 1s 轨道。 -
2s²: Two electrons fill the spherical
2s orbital.
2s²:两个电子填满球形的 2s 轨道。 -
2p²: Now, let’s look at the
p-sublevel. Carbon has two electrons to place in its2p orbitals. According to Hund’s Rule:
2p²:接下来看 p-亚层。碳有两个电子需填充到 2p 轨道中,根据洪特规则:- The first electron occupies, for example, the
2px orbital.
第一个电子占据(如)2px 轨道。 - The second electron moves into another
2p orbital– say, the2py orbital.
第二个电子进入另一个 2p 轨道(如 2py 轨道)。 - These two electrons do not pair up; instead, each occupies its own
2p orbitalto maximize separation and minimize energy.
这两个电子不会配对,而是各自占据一个 2p 轨道,以实现最大距离分离并降低能量。
- The first electron occupies, for example, the
This specific electron arrangement in p-orbitals has a major influence on an atom’s chemical behavior – particularly its ability to form chemical bonds and its overall molecular geometry.
p-轨道中这种特定的电子排布方式,对原子的化学性质(尤其是成键能力和整体分子几何结构)有着重要影响。
As we move beyond these directional “dumbbells,” we’re ready to discover even more intricate and dynamic “homes” for electrons.
当我们越过这些定向“哑铃”后,接下来将探索更复杂、更具动态性的电子“居所”。
After exploring the simplicity of the s-sublevel and the unique peanut-like structure of the p-sublevel, we’re now prepared to step into a realm of even greater complexity – one with profound chemical significance.
在探索了 s-亚层的简洁性与 p-亚层独特的花生状结构后,我们已准备好进入一个更复杂、且具有深远化学意义的领域。
Shaping the Spectrum: Unveiling the Dynamic d-sublevel and the Marvels of Transition Metals
塑造光谱:揭示动态 d-亚层和过渡金属的奇妙之处
As we ascend to the third Energy Level of an atom,a fascinating and crucial new player emerges on the scene: the d-sublevel.This particular sublevel isn’t just an arbitrary addition to our atomic model; it’s the very heart of understanding a captivating and incredibly useful group of elements known as transition metals.Without the d-sublevel,the vibrant colors,catalytic powers,and diverse properties of these elements would remain a mystery.
当我们上升到原子的第三个能量级时,一个有趣且关键的新角色出现在舞台上:d-亚层。这个特定的亚层不仅仅是原子模型的一个随意添加;它是理解一个迷人且极其有用的元素群——过渡金属的核心。没有 d-亚层,这些元素的鲜艳颜色、催化能力以及多样化的性质将仍然是一个谜。
The Appearance and Importance of the d-sublevel
d-亚层的出现及其重要性
The d-sublevel makes its first appearance starting from the third principal energy level (n = 3).While the first energy level only has an s-sublevel,and the second has s and p,the third level introduces s,p,and d.This addition is paramount because the unique characteristics of the transition metals – that large block of elements nestled in the middle of the Periodic Table – are almost entirely dictated by how their d-sublevels are filled with electrons.Understanding the d-sublevel is key to unlocking the secrets of elements like iron,copper,gold,and zinc.
d-亚层从第三主能量级(n = 3)开始首次出现。虽然第一能量级只有 s-亚层,第二能量级有 s 和 p,但第三能量级引入了 s、p 和 d。这一添加至关重要,因为过渡金属的独特特性——周期表中间那一整块元素——几乎完全由它们的 d-亚层如何被电子填充所决定。理解 d-亚层是解开像铁、铜、金和锌这样的元素秘密的关键。
Intricate Shapes: The Five d-Atomic Orbitals
复杂的形状:五个 d-原子轨道
If the p-sublevel introduced us to directional,dumbbell-shaped orbitals,the d-sublevel takes complexity to a whole new level.It comprises a total of five distinct Atomic Orbitals,each with an elaborate and specific three-dimensional shape.Let’s explore these more intricate forms:
如果 p-亚层向我们介绍了有方向的、哑铃形的轨道,那么d-亚层将复杂性提升到了一个全新的水平。它包含总共五个不同的原子轨道,每个轨道都有一个复杂且特定的三维形状。让我们来探索这些更复杂的形式:
-
Four Clover-Leaf Like Orbitals: Imagine combining two p-orbitals at right angles to each other.Four of the d-orbitals have a shape often described as “clover-leaf” or “four-lobed,” resembling a four-leaf clover.These orbitals lie in specific planes,pointing their lobes between or along the x,y,and z axes (dxy,dxz,dyz,and dx²-y²).
四个三叶草叶状轨道:想象一下将两个 p-轨道以直角组合在一起。四个 d-轨道的形状通常被描述为“三叶草形”或“四叶形”,类似于四叶草。这些轨道位于特定的平面上,它们的叶片指向 x、y 和 z 轴之间或沿轴的方向(dxy、dxz、dyz 和 dx²-y²)。 -
One Dumbbell with a Ring Orbital: The fifth d-orbital (dz²) is unique.It consists of two lobes resembling a dumbbell along the z-axis,but crucially,it also features a doughnut-shaped ring of electron density wrapped around the center of these two lobes in the xy-plane.It’s a truly distinctive and somewhat whimsical shape!
一个带环的哑铃轨道:第五个 d-轨道(dz²)是独一无二的。它由两个沿 z 轴方向的类似哑铃的叶片组成,但关键的是,它还在 xy 平面上围绕这两个叶片的中心有一个类似甜甜圈的电子密度环。这是一个真正独特且有点异想天开的形状!
These complex shapes mean that d-electrons can interact in various ways,contributing to the diverse chemistry of their elements.
这些复杂的形状意味着 d-电子可以以各种方式相互作用,从而促成了它们元素的多样化化学性质。
Electron Capacity: Holding Up to Ten
电子容量:最多可容纳十个
Since the d-sublevel consists of five individual Atomic Orbitals,and each atomic orbital can hold a maximum of two electrons (thanks to Wolfgang Pauli’s exclusion principle),we can easily calculate its total electron capacity.These five orbitals can collectively accommodate up to ten electrons.This capacity of ten electrons is a critical number when we look at the arrangement of elements on the Periodic Table.
由于d-亚层由五个独立的原子轨道组成,而每个原子轨道最多可以容纳两个电子(多亏了泡利不相容原理),我们可以轻松计算出它的总电子容量。这五个轨道总共可以容纳多达十个电子。当我们在周期表中查看元素的排列时,这个十电子的容量是一个关键数字。
The d-sublevel and the Transition Metals Block
d-亚层与过渡金属块
The sequential filling of these ten d-electrons is what defines the entire block of transition metals on the Periodic Table.As we move across a period within this block,electrons are progressively added to the d-sublevel.This process has profound implications:
这十个 d-电子的顺序填充定义了周期表中整个过渡金属块。当我们在这个块内穿过一个周期时,电子会逐渐添加到 d-亚层中。这个过程有着深远的影响:
-
Diverse Chemical Properties: The d-electrons are often the ones involved in chemical bonding,leading to the characteristic variable oxidation states (meaning they can form ions with different charges) of transition metals.
多样的化学性质:d-电子通常是参与化学键形成的电子,这导致了过渡金属的特征性可变氧化态(意味着它们可以形成不同电荷的离子)。 -
Vibrant Colors: Many compounds involving transition metals are brightly colored (think of the blue of copper sulfate or the green of nickel compounds).This is due to the way their d-electrons absorb and emit light when they jump between slightly different energy levels within the d-orbitals.
鲜艳的颜色:许多涉及过渡金属的化合物颜色鲜艳(想想硫酸铜的蓝色或镍化合物的绿色)。这是由于它们的 d-电子在 d-轨道内稍微不同的能量级之间跳跃时吸收和发射光的方式。 -
Catalytic Activity: Transition metals and their compounds are frequently used as catalysts in industrial processes (e.g.,iron in the Haber-Bosch process).Their partially filled d-orbitals provide sites for other molecules to bind,facilitating chemical reactions.
催化活性:过渡金属及其化合物经常被用作工业过程中的催化剂(例如,哈伯-博施过程中的铁)。它们部分填充的 d-轨道为其他分子提供了结合位点,促进了化学反应。
In essence,the d-sublevel is where chemistry truly begins to show its dynamic and colorful side,laying the foundation for many of the materials and processes essential to our world.
总之,d-亚层是化学真正开始展现其动态和多彩一面的地方,为许多对我们世界至关重要的材料和过程奠定了基础。
Our exploration of electron housing within the atom continues,as we next prepare to venture even further out,to the most expansive and fundamental f-sublevel.
我们对原子内电子居所的探索仍在继续,接下来我们将准备进一步深入,进入最广阔且基础的 f-亚层。
After navigating the intricate landscape of the d-sublevel,we’re ready to venture even deeper into the atomic structure to uncover its most complex electron dwelling.
在经历了 d-亚层复杂的地形之后,我们已经准备好深入原子结构,去揭开它最复杂的电子居所的面纱。
The Ultimate Frontier: Decoding the Far-Out f-sublevel
最终边疆:解码遥远的 f-亚层
Welcome to the f-sublevel,truly the most complex and ‘far-out’ sublevel we’ll encounter in introductory chemistry.It makes its grand entrance starting from the fourth Energy Level (n = 4) and beyond.Think of it as the deepest,most intricate chamber within the atom’s grand design,holding secrets to some unique elements.
欢迎来到 f-亚层,这是我们在基础化学中将遇到的最复杂且最“遥远”的亚层。它从第四能量级(n = 4)及更高开始隆重登场。可以将其视为原子宏伟设计中最深处、最复杂的腔室,它保存着一些独特元素的秘密。
Complex Shapes You Don’t Need to Memorize (Yet!)
你不需要记忆的复杂形状
Unlike the simple spheres of the s-sublevel or the familiar dumbbells of the p-sublevel,the f-sublevel orbitals are incredibly intricate and varied in their shapes.Imagine multi-lobed structures that look like complex flowers or even more abstract art! These shapes are so detailed that,for a beginner,the crucial takeaway here isn’t to visualize their exact forms.Instead,it’s enough to know that there are seven distinct f-orbitals.Each of these seven orbitals occupies a unique region of space around the nucleus,offering a specific home for electrons.
与 s-亚层的简单球体或 p-亚层熟悉的哑铃不同,f-亚层轨道的形状极其复杂且各不相同。想象一下像复杂的花朵甚至更抽象的画作一样的多叶片结构!这些形状如此复杂,对于初学者来说,这里的关键收获不是去想象它们的确切形状。相反,只要知道有七个不同的 f-轨道就足够了。这七个轨道中的每一个都占据着原子核周围独特的空间区域,为电子提供了一个特定的家园。
A Massive Electron Capacity
巨大的电子容量
With seven unique atomic orbitals available,the f-sublevel boasts a truly massive electron capacity.Each of these seven orbitals,just like those in s,p,and d sublevels,can comfortably house a pair of electrons.This means the f-sublevel can accommodate a grand total of fourteen (7 orbitals × 2 electrons/orbital) electrons! This immense capacity plays a vital role in the properties of some truly fascinating elements,setting them apart from the more common ones.
由于有七个独特的原子轨道可供使用,f-亚层拥有真正巨大的电子容量。这七个轨道中的每一个,就像 s、p 和 d 亚层中的轨道一样,都可以舒适地容纳一对电子。这意味着 f-亚层总共可以容纳**十四个(7 个轨道 × 每个轨道 2 个电子)电子!**这种巨大的容量在一些真正迷人的元素的性质中扮演着重要的角色,使它们与更常见的元素区别开来。
The Lanthanides and Actinides: f-block Elements
镧系元素和锕系元素:f-区元素
Where do we see this impressive f-sublevel in action on the Periodic Table? Look to the two distinct rows often placed below the main body of the table: the Lanthanide and Actinide series.These elements,sometimes called the ‘inner transition metals,’ are precisely where atoms are busy filling their f-sublevels.The Lanthanides,specifically,are filling the 4f-sublevel,while the Actinides are filling the 5f-sublevel.Their unique placement and chemical behavior highlight the special role these deeply buried f-electrons play in defining their atomic properties.
在周期表中,我们可以在哪里看到这个令人印象深刻的 f-亚层发挥作用呢?看看通常放在表格主体下方的两行不同的元素:镧系元素和锕系元素。这些元素有时被称为“内过渡金属”,正是原子忙于填充它们的 f-亚层的地方。具体来说,镧系元素正在填充 4f-亚层,而锕系元素正在填充 5f-亚层。它们独特的排列和化学行为突出了这些深埋的 f-电子在定义它们的原子性质中所起的特殊作用。
Understanding these intricate sublevels,from s to f,is the crucial groundwork for our next step: learning how to precisely map out an atom’s electron arrangement.
理解从 s 到 f 这些复杂的亚层,是我们下一步的关键基础:学习如何精确地绘制出原子的电子排列。
Having journeyed through the intricacies of the s,p,d,and f sublevels,understanding their shapes and electron capacities,it’s time to bring all that fascinating knowledge together.
在经历了 s、p、d 和 f 亚层的复杂性,理解了它们的形状和电子容量之后,是时候将所有这些迷人的知识整合在一起了。
The Atomic Architect: Crafting Electron Configurations
原子建筑师:构建电子构型
You’ve explored the unique neighborhoods where electrons reside.Now,imagine yourself as an atomic architect,tasked with creating the blueprint for any atom,meticulously detailing where each of its electrons can be found.This blueprint is what we call an Electron Configuration,and it’s a powerful tool for understanding how atoms interact and behave.
你已经探索了电子居住的独特街区。现在,想象你自己是一名原子建筑师,任务是为任何原子创建蓝图,详细说明每个电子的位置。这个蓝图就是我们所说的电子构型,它是理解原子如何相互作用和行为的强大工具。
The Guiding Principles of Electron Arrangement
电子排列的指导原则
To accurately build an electron configuration,we follow three fundamental rules,acting as our architectural guidelines.These principles ensure that electrons settle into the most stable and lowest-energy arrangements possible within an atom.
为了准确地构建电子构型,我们遵循三个基本规则,这些规则作为我们的建筑设计指南。这些原则确保电子在原子内尽可能稳定且处于最低能量的排列。
The Aufbau Principle: Building from the Ground Up
奥布夫原理:从地面开始建造
The German word “Aufbau” means “building up.” This principle states that electrons will always occupy the lowest energy orbitals available before moving to higher energy ones.Think of it like filling seats in a theater: you fill the front rows first before moving to the back.This makes perfect sense,as electrons naturally seek the most stable,lowest-energy state.
德语中的“Aufbau”意味着“建造”。这个原理指出,电子总是会先占据可用的最低能量轨道,然后才会移动到更高能量的轨道。这就好比在剧院里填满座位:你会先填满前排座位,然后才会移到后排。这完全符合逻辑,因为电子自然会寻找最稳定、最低能量的状态。
The Pauli Exclusion Principle: Every Electron Has Its Space
泡利不相容原理:每个电子都有自己的空间
Named after Wolfgang Pauli,this principle tells us that each orbital can hold a maximum of two electrons,and these two electrons must have opposite spins (one “up” and one “down”).This is crucial because it ensures that no two electrons in an atom can have the exact same set of quantum numbers,giving each electron a unique “address.”
以沃尔夫冈·泡利命名的这一原理告诉我们,每个轨道最多可以容纳两个电子,而且这两个电子必须具有相反的自旋(一个“向上”和一个“向下”)。这一点至关重要,因为它确保了一个原子中的两个电子不能拥有完全相同的量子数集合,从而给每个电子一个独特的“地址”。
Hund’s Rule: Sharing the Sublevel Love
洪特规则:共享亚层的爱
When you have multiple orbitals of equal energy within a sublevel (like the three p-orbitals or five d-orbitals),Hund’s Rule dictates how electrons will fill them.It states that electrons will occupy each orbital within a sublevel singly before any orbital gets a second electron.And,all these singly occupied electrons will have the same spin.Imagine friends finding seats on a bus: they’ll each take an empty seat before sitting next to someone.
当你在一个亚层中有多个能量相等的轨道(比如三个 p-轨道或五个 d-轨道)时,洪特规则决定了电子将如何填充它们。它指出,电子将在任何一个轨道获得第二个电子之前,先单独占据亚层中的每个轨道。而且,所有这些单独占据的电子将具有相同的自旋。想象一下朋友们在公交车上找座位:他们会在坐在别人旁边之前,先各自找一个空座位。
The Orbital Filling Roadmap: Navigating the Energy Levels
轨道填充路线图:导航能量级
Keeping the Aufbau Principle in mind,it’s helpful to visualize the order in which orbitals are filled.While you might intuitively think 1s,2s,2p,3s,3p,3d,4s…the actual energy levels can sometimes overlap,particularly with d and f orbitals.The following diagram,often called a diagonal rule chart,provides a handy roadmap for the filling order:
记住奥布夫原理,有助于可视化轨道填充的顺序。虽然你可能会直观地认为是 1s、2s、2p、3s、3p、3d、4s……但实际的能量级有时会重叠,特别是 d 和 f 轨道。下面的图表,通常称为对角线规则图,为填充顺序提供了一个方便的路线图:
| Orbital Filling Order (Diagonal Rule) |
|---|
| 1s |
| 2s |
| 2p |
| 3s |
| 3p |
| 4s |
| 3d |
| 4p |
| 5s |
| 4d |
| 5p |
| 6s |
| 4f |
| 5d |
| 6p |
| 7s |
| 5f |
| 6d |
| 7p |
To use this,follow the arrows diagonally from top right to bottom left.Start at 1s,then 2s,then 2p,then 3s,then 3p,then 4s,then 3d,and so on.
使用这个图表时,沿着从右上到左下的对角线方向跟随箭头。从 1s 开始,然后是 2s,接着是 2p,然后是 3s,接着是 3p,然后是 4s,接着是 3d,依此类推。
Let’s Build One Together: Phosphorus §
让我们一起构建一个:磷(P)
Let’s put these rules into action and write the electron configuration for Phosphorus §.
让我们将这些规则付诸行动,写出**磷(P)**的电子构型。
1.Find the Atomic Number: Phosphorus § has an atomic number of 15.This means a neutral phosphorus atom has 15 protons and,crucially for our configuration,15 electrons.
找到原子序数:磷(P)的原子序数为 15。这意味着一个中性的磷原子有 15 个质子,对我们构型至关重要的是,它有15 个电子。
2.Start Filling from the Lowest Energy (Aufbau):
从最低能量开始填充(奥布夫):
- 1s orbital: Can hold 2 electrons.We fill it:
1s²(2 electrons used,13 remaining)
1s 轨道:可以容纳 2 个电子。我们将其填满:1s²(已用 2 个电子,剩余 13 个) - 2s orbital: Next lowest.Can hold 2 electrons.We fill it:
2s²(4 electrons used,11 remaining)
2s 轨道:接下来最低的。可以容纳 2 个电子。我们将其填满:2s²(已用 4 个电子,剩余 11 个) - 2p sublevel: Next,the 2p sublevel.It has three orbitals,each holding 2,for a total of 6 electrons.We fill it:
2p⁶(10 electrons used,5 remaining)
2p 亚层:接下来是 2p 亚层。它有三个轨道,每个轨道可以容纳 2 个电子,总共可以容纳 6 个电子。我们将其填满:2p⁶(已用 10 个电子,剩余 5 个) - 3s orbital: Next lowest.Can hold 2 electrons.We fill it:
3s²(12 electrons used,3 remaining)
3s 轨道:接下来最低的。可以容纳 2 个电子。我们将其填满:3s²(已用 12 个电子,剩余 3 个) - 3p sublevel: Next,the 3p sublevel.It has three orbitals,capable of holding 6 electrons total.We only have 3 electrons left.According to Hund’s Rule,we’ll place one electron in each of the three 3p orbitals before pairing any up:
3p³(15 electrons used,0 remaining)
3p 亚层:接下来是 3p 亚层。它有三个轨道,总共可以容纳 6 个电子。我们只剩下 3 个电子。根据洪特规则,我们会先在三个 3p 轨道中各放置一个电子,然后再将它们配对:3p³(已用 15 个电子,剩余 0 个)
3.The Full Electron Configuration for Phosphorus §: By combining these steps,the complete electron configuration for Phosphorus is: 1s² 2s² 2p⁶ 3s² 3p³
3.磷(P)的完整电子构型:通过合并这些步骤,磷的完整电子构型是:1s² 2s² 2p⁶ 3s² 3p³
This notation tells us exactly how the 15 electrons of a phosphorus atom are distributed among its orbitals.
这种表示法准确地告诉我们磷原子的 15 个电子是如何在其轨道中分布的。
The Outer Shell Story: Unveiling Valence Electrons
外层壳的故事:揭示价电子
Look closely at the final electron configuration for Phosphorus: 1s² 2s² 2p⁶ 3s² 3p³.The electrons in the outermost principal energy level are the most important for determining an element’s chemical behavior.These are called Valence Electrons.
仔细看看磷的最终电子构型:1s² 2s² 2p⁶ 3s² 3p³。最外层主能量级中的电子对于确定元素的化学行为最为重要。这些被称为价电子。
For Phosphorus,the highest principal energy level reached is n=3.The electrons in this level are those in the 3s and 3p sublevels.
对于磷来说,达到的最高主能量级是n=3。这一层中的电子是3s和3p亚层中的电子。
3s²contributes 2 valence electrons.
3s²贡献了 2 个价电子。3p³contributes 3 valence electrons.
3p³贡献了 3 个价电子。- Total Valence Electrons for Phosphorus = 2 + 3 = 5.
磷的总价电子数 = 2 + 3 = 5。
These 5 valence electrons are the ones Phosphorus will use to form bonds with other atoms,gain or lose electrons,and dictate its reactivity.Mastering electron configurations is truly the key to unlocking an element’s chemical personality!
这 5 个价电子是磷用来与其他原子形成化学键、获得或失去电子以及决定其活性的电子。掌握电子构型确实是解锁元素化学个性的关键!
Now that you’ve navigated the intricate world of electron configurations,charting the course for atomic structure,you’re ready to see how this knowledge forms the bedrock of our understanding of chemical properties.
现在你已经成功地穿过了电子构型的复杂世界,为原子结构绘制了路线图,你已经准备好看到这些知识如何构成了我们对化学性质理解的基础。
Now that you’ve successfully navigated the intricate rules for assigning electrons to their proper places,it’s time to appreciate the power of that knowledge.
现在你已经成功地穿过了为电子分配适当位置的复杂规则,是时候欣赏这些知识的力量了。
Beyond the Basics: Charting Your Course from s,p,d,f to Chemical Mastery
超越基础:从 s、p、d、f 到化学精通的路线图
You’ve done it! After diving deep into the world of electron configurations,you’ve successfully built a solid foundation in understanding the four primary Atomic Sublevels: s,p,d,and f.This is a significant achievement and a crucial step in unraveling the mysteries of chemistry.Congratulations! You now possess a key piece of the puzzle that explains how atoms are structured and how they behave.
你做到了!深入电子构型的世界之后,你成功地为理解四个主要的原子亚层:s、p、d 和 f,奠定了坚实的基础。这是一个重要的成就,也是解开化学奥秘的关键一步。恭喜你!你现在拥有了解释原子结构和行为的拼图的关键一块。
What You’ve Mastered So Far
你已经掌握的内容
Let’s quickly refresh your memory on the key distinctions you’ve learned.Each sublevel isn’t just a letter; it represents a unique spatial region around the nucleus where electrons are likely to be found,known as an Orbital Shape.You’ve grasped both their distinct shapes and their specific Electron Capacity:
让我们快速回顾一下你已经学到的关键区别。每个亚层不仅仅是一个字母;它代表一个独特的空间区域,即轨道形状,电子可能在原子核周围找到。你已经掌握了它们独特的形状和特定的电子容量:
-
s sublevel: This is the simplest,with a spherical shape.It always contains one orbital and can hold a maximum of 2 electrons.
s 亚层:这是最简单的,呈球形。它始终包含一个轨道,最多可以容纳 2 个电子。 -
p sublevel: With its familiar dumbbell-shaped orbitals,the p sublevel consists of three orbitals oriented along the x,y,and z axes,capable of housing up to 6 electrons.
p 亚层:凭借其熟悉的哑铃形轨道,p 亚层由沿 x、y 和 z 轴方向排列的三个轨道组成,总共可以容纳多达 6 个电子。 -
d sublevel: More complex,featuring cloverleaf-like shapes (with one unique shape resembling a dumbbell with a donut),this sublevel comprises five orbitals,accommodating a total of 10 electrons.
d 亚层:更加复杂,呈现出三叶草叶状的形状(其中一种独特形状类似于带甜甜圈的哑铃),这个亚层包含五个轨道,总共可以容纳 10 个电子。 -
f sublevel: Exhibiting even more intricate shapes,the f sublevel is made up of seven orbitals,with the capacity to hold an impressive 14 electrons.
f 亚层:呈现出更加复杂的形状,f 亚层由七个轨道组成,能够容纳多达 14 个电子。
You now understand not only the unique physical arrangements of electrons within these sublevels but also the strict limits on how many electrons each can contain,a concept fundamental to all of chemistry.
你不仅理解了这些亚层中电子的独特物理排列,还理解了每个亚层可以容纳的电子数量的严格限制,这是化学学科中的一个基本概念。
Your Gateway to Deeper Understanding
深入理解的门户
This isn’t just abstract knowledge; it’s a powerful toolkit.Your firm grasp of Atomic Structure,particularly the organization of electrons within these sublevels,is the bedrock upon which much of chemistry is built.Think of it as learning the alphabet before you can read a book.This understanding is a critical stepping-stone,essential for comprehending how atoms interact,why certain elements are highly reactive while others are inert,and the fascinating trends that govern the entire Periodic Table.
这不仅仅是抽象的知识;它是一个强大的工具包。你对原子结构的牢固掌握,特别是这些亚层中电子的排列,是化学学科创立的基石。可以把它想象成在读书之前先学习字母表。这种理解是一个关键的垫脚石,对于理解原子如何相互作用、为什么某些元素高度活跃而其他元素则不活跃,以及整个周期表的有趣趋势至关重要。
You’re now equipped to see patterns and predict behaviors that once seemed random.This foundational insight will make future chemical concepts much more intuitive and exciting to explore.You’ve truly opened the door to a deeper appreciation of the chemical world around us!
你现在能够看到曾经看似随机的模式并预测行为。这种基础的洞察力将使未来的化学概念更加直观和令人兴奋。你真的为我们周围化学世界的更深入理解打开了大门!
What’s Next on Your Chemical Journey?
你化学之旅的下一步是什么?
With this vital knowledge under your belt,you’re perfectly positioned to venture into more advanced topics.A logical next step is to explore how these atomic sublevels and orbitals combine and reorganize when atoms bond.
带着这些重要的知识,你完全有能力探索更高级的主题。一个合理的下一步是探索这些原子亚层和轨道在原子结合时是如何组合和重新排列的。
You might delve into the concept of orbital hybridization,where atomic orbitals mix to form new,hybrid orbitals that allow for more stable and diverse bonding arrangements.Alternatively,you could focus on valence electrons – those outermost electrons residing in the s and p sublevels – and discover their crucial role in creating chemical bonds.Understanding how these electrons are shared or transferred between atoms is fundamental to explaining the existence of molecules and compounds that make up our world.
你可能会深入研究轨道杂化的概念,即原子轨道混合形成新的杂化轨道,从而允许更稳定和多样化的键合排列。或者,你可以专注于价电子——那些位于 s 和 p 亚层的最外层电子——并发现它们在形成化学键中所起的关键作用。理解这些电子是如何在原子之间共享或转移的,对于解释构成我们世界的分子和化合物的存在至关重要。
As you continue your exploration,you’ll uncover how these fundamental principles translate into the diverse and dynamic world of chemical interactions.
随着你继续探索,你会发现这些基本原理是如何转化为多样化和动态的化学相互作用世界的。
Frequently Asked Questions About The 4 Energy Sublevels Explained: A Simple s,p,d,f Guide
关于四个能量亚层的常见问题:一个简单的 s、p、d、f 指南
What do s,p,d,and f refer to in atomic structure?
在原子结构中,s、p、d 和 f 指的是什么?
These letters denote the four primary types of sublevels of energy found within an atom’s electron shells.Each sublevel corresponds to a specific shape of electron orbital and has a distinct energy associated with it,helping to organize where electrons reside.
这些字母表示原子电子壳层中发现的四种主要能量亚层类型。每个亚层对应于特定形状的电子轨道,并且与之相关联的特定能量有助于组织电子的居住位置。
How many electrons can each of these sublevels accommodate?
这些亚层各自可以容纳多少个电子?
Each type of sublevels of energy has a fixed maximum capacity for electrons.The s sublevel can hold up to 2 electrons,p can hold 6,d can hold 10,and the f sublevel can accommodate 14 electrons.This capacity is determined by the number of orbitals within each sublevel.
每种能量亚层类型都有固定的电子最大容量。s 亚层最多可以容纳 2 个电子,p 可以容纳 6 个,d 可以容纳 10 个,而 f 亚层可以容纳 14 个电子。这种容量是由每个亚层中的轨道数量决定的。
What is the distinction between an energy level and a sublevel?
能量级和亚层之间的区别是什么?
A principal energy level (or electron shell) represents a broad region of space around the nucleus.Within each of these main energy levels,there are more specific classifications called sublevels of energy (s,p,d,f).These sublevels further differentiate electrons based on their orbital shapes and slightly varying energies within that principal shell.
主能量级(或电子壳层)代表原子核周围的一个广阔空间区域。在这些主能量级中,有更具体的分类称为能量亚层(s、p、d、f)。这些亚层进一步根据电子的轨道形状和在主壳层内略有不同的能量来区分电子。
Why is it important to understand the s,p,d,f sublevels?
为什么理解 s、p、d、f 亚层很重要?
Understanding the sublevels of energy is fundamental to predicting and explaining an atom’s chemical properties and behavior.They dictate how electrons are arranged in an atom (electron configuration),which directly influences its reactivity,its ability to form bonds,and many other chemical characteristics.
理解能量亚层是预测和解释原子的化学性质和行为的基础。它们决定了原子中电子的排列(电子构型),这直接影响了它的活性、形成化学键的能力以及许多其他化学特性。
Congratulations on mastering the s,p,d,and f sublevels! You’ve successfully journeyed from the simple sphere of the s-orbital to the complex shapes of the d and f-sublevels.You now possess a powerful foundational knowledge of atomic structure.
恭喜你掌握了 s、p、d 和 f 亚层! 你已经成功地从简单的 s-轨道的球形走到了复杂的 d 和 f-亚层的形状。你现在拥有强大的基础原子结构知识。
Remember the key takeaways: each sublevel has a unique shape and a specific electron capacity,governed by the rules of quantum mechanics.This isn’t just abstract theory; it’s the essential framework needed to understand chemical bonding,reactivity,and the elegant trends seen across the Periodic Table.Your next step? Consider exploring how these orbitals mix to form chemical bonds through hybridization or diving deeper into the role of valence electrons in chemical reactions.The atomic world is now yours to explore!
记住关键要点:每个亚层都有一个独特的形状和特定的电子容量,由量子力学的规则控制。这不仅仅是抽象的理论;这是理解化学键、活性以及整个周期表中看到的优雅趋势所必需的基本框架。你的下一步?考虑探索这些轨道是如何通过杂化形成化学键的,或者更深入地研究价电子在化学反应中的作用。原子世界现在由你来探索!
量子数(n,l,mₗ,mₛ)和亚层(s,p,d,f)
一、量子数(n,l,mₗ,mₛ)的名称起源与含义
The origins of the quantum numbers (n, l, mₗ, mₛ) are English, and slightly messy.
量子数(n,l,mₗ,mₛ)的名称起源于英语,且含义关联较为零散。
-
Principal Quantum Number (n,主量子数)
“n” is a common algebraic convention for an integer variable or constant, representing the “energy level” of electrons (e.g., n=1, 2, 3… correspond to the first, second, third energy levels respectively).
“n”是整数变量或常数的常见代数表示惯例,代表电子的“能量级”(例如 n=1、2、3……分别对应第一、第二、第三能量级)。
n = 1 , 2 , 3 , … n = 1, 2, 3, \ldots n=1,2,3,… -
Azimuthal (Angular Momentum) Quantum Number (l,角量子数)
“l” is a typical third choice in algebraic notation (after common variables like x, y) and has angular momentum connotations in other physical applications. It determines the “shape of orbitals” and ranges from 0 to n-1 (for Coulombic systems like hydrogen atoms); for example, l=0 corresponds to s orbitals, l=1 to p orbitals, etc. Note that this range (0 to n-1) is not universal—for spherical harmonic oscillators, l ranges from 0 to n with a step of 2 instead of 1.
“l”是代数符号中典型的“第三选择”(仅次于常用变量 x、y),在其他物理应用中带有角动量含义。它决定“轨道形状”,取值范围为 0 到 n-1(适用于氢原子等库仑系统);例如 l=0 对应 s 轨道,l=1 对应 p 轨道等。需注意该取值范围并非通用——对于球形谐振子,l 的取值范围为 0 到 n,且步长为 2(而非 1)。
l = 0 , 1 , 2 , … , n − 1 l = 0, 1, 2, \ldots, n - 1 l=0,1,2,…,n−1 -
Magnetic Quantum Number (mₗ,磁量子数)
“m” stands for “magnetic” (reflecting its association with magnetic fields), and the subscript “l” indicates it is related to the azimuthal quantum number l. It describes the “spatial orientation of orbitals” and ranges from -l to +l (e.g., if l=1, mₗ can be -1, 0, +1, corresponding to px, py, pz orbitals respectively).
“m”代表“magnetic(磁性)”(体现其与磁场的关联),下标“l”表示它与角量子数 l 相关,描述“轨道的空间取向”,取值范围为 -l 到 +l(例如 l=1 时,mₗ 可取值 -1、0、+1,分别对应 px、py、pz 轨道)。
m l = − l , − l + 1 , … , 0 , … , l − 1 , l m_l = -l, -l + 1, \ldots, 0, \ldots, l - 1, l ml=−l,−l+1,…,0,…,l−1,l -
Spin Quantum Number (mₛ,自旋量子数)
“s” stands for “spin” (referring to the intrinsic spin of electrons), and the subscript “s” distinguishes it from the magnetic quantum number mₗ. It describes the “spin direction of electrons” and has only two possible values: +1/2 (spin up) and -1/2 (spin down), which follows the Pauli Exclusion Principle (no two electrons in an atom can have the same set of four quantum numbers).
“s”代表“spin(自旋)”(指电子的内禀自旋属性),下标“s”用于与磁量子数 mₗ 区分,描述“电子的自旋方向”,仅有两个可能取值:+1/2(自旋向上)和 -1/2(自旋向下),这一特性遵循泡利不相容原理(原子中无两个电子的四个量子数完全相同)。
m s = + 1 2 , − 1 2 m_s = +\frac{1}{2}, -\frac{1}{2} ms=+21,−21
二、亚层(s,p,d,f)的命名起源与后续规则
1. 起源:基于早期光谱观测的历史分类
The letters s, p, d, f originate from the analysis of emission spectra of hydrogen atoms and alkali metals (e.g., sodium, lithium). Early spectroscopists classified observed spectral line series by their qualitative characteristics, and these names were later adopted to label orbitals/sublevels:
s、p、d、f 四个字母起源于对氢原子和碱金属(如钠、锂)发射光谱的分析。早期光谱学家根据观测到的光谱线系列的定性特征进行分类,这些名称后来被沿用为轨道/亚层的标识:
-
s (Sharp)
Named after the “sharp series” of spectral lines—these lines appeared narrow and well-defined. Later, it was found that the sharp series arises from electron transitions from p orbitals to s orbitals (np → s).
源于“sharp series(尖锐线系)”——这类光谱线表现为窄且清晰的形态。后续研究发现,尖锐线系来自电子从 p 轨道向 s 轨道的跃迁(np → s)。 -
p (Principal)
Named after the “principal series”—the most intense and vivid lines in the spectrum. It corresponds to electron transitions from s orbitals to p orbitals (ns → p).
源于“principal series(主线系)”——光谱中强度最高、最鲜明的谱线系列,对应电子从 s 轨道向 p 轨道的跃迁(ns → p)。 -
d (Diffuse)
Named after the “diffuse series”—lines that were broad and ill-defined. It is associated with transitions from d orbitals to p orbitals (nd → p).
源于“diffuse series(漫线系)”——谱线表现为宽且模糊的形态,对应电子从 d 轨道向 p 轨道的跃迁(nd → p)。 -
f (Fundamental/Faint)
The most debated origin, but “fundamental” is overwhelmingly accepted (not “fine” as some claim). It comes from the “fundamental series” (also called the Bergmann series, discovered by Arno Bergmann in 1907), initially thought to be the “basic series” for each element. Early references (e.g., 1911 studies) confirm “F” stands for “fundamental.” This series arises from transitions from f orbitals to d orbitals (nf → d), and some early observations noted its lines were faint—hence the occasional confusion with “faint.”
起源存在争议,但“fundamental(基本)”是公认的主流含义(而非部分说法中的“fine(精细)”)。它源于“fundamental series(基本线系,又称伯格曼线系,由 Arno Bergmann 于 1907 年发现)”,最初被认为是每种元素的“基础谱线系列”,早期文献(如 1911 年的研究)明确“F”代表“fundamental”。该线系对应电子从 f 轨道向 d 轨道的跃迁(nf → d),因早期观测中部分谱线较微弱,偶尔会与“faint(微弱)”混淆。
2. 命名规则的延续与变化
-
Beyond f (f 之后的亚层)
After s, p, d, f, subsequent sublevels follow alphabetical order, skipping the letter “E” (to avoid confusion with other scientific notations). The sequence is: s, p, d, f, g, h, i… These later letters (g, h, etc.) have no historical spectroscopic meanings—they are merely sequential labels.
s、p、d、f 之后的亚层按字母顺序命名,但跳过字母“E”(避免与其他科学符号混淆),顺序为:s、p、d、f、g、h、i……这些后续字母(g、h 等)无历史光谱学含义,仅为顺序标识。 -
Why not numbers?
Using letters (s, p, d, f…) instead of number series for sublevels (while energy levels use n=1, 2, 3…) avoids confusion between different atomic structure parameters. Although the original spectroscopic meanings of s, p, d, f are no longer directly relevant to orbital properties (e.g., an s orbital is not “sharp”), the names have been retained due to historical convention.
亚层使用字母(s、p、d、f……)而非数字标识(能量级已使用 n=1、2、3……),是为了避免不同原子结构参数的混淆。尽管 s、p、d、f 的原始光谱学含义与轨道属性已无直接关联(例如 s 轨道并不“尖锐”),但这些名称因历史惯例被保留至今。
三、d 轨道的空间形态(以 dₓᵧ、dₓz、dᵧz、dₓ²₋ᵧ²、d_z² 为例)
d
z
2
d_{z^2}
dz2 and other d orbitals belong to atomic orbitals with azimuthal quantum number
l
=
2
l=2
l=2 (corresponding to
n
≥
3
n \geq 3
n≥3), and there are five spatial orientations (magnetic quantum number
m
l
m_l
ml: -2, -1, 0, +1, +2), each with a distinct shape:
d
z
2
d_{z^2}
dz2 与其他 d 轨道均属于角量子数
l
=
2
l=2
l=2 的原子轨道(对应
n
≥
3
n \geq 3
n≥3),共有五种空间取向(磁量子数
m
l
m_l
ml:-2、-1、0、+1、+2),每种取向的轨道形态不同:
-
d x y d_{xy} dxy
Electron density is mainly distributed in the x-y plane, extending along directions 45° to the x and y axes (petal-like shape). The nucleus is at the center of the plane, and the x/y axes are nodal planes (where electron density is zero).
电子密度主要分布在 x-y 平面内,沿与 x 轴、y 轴成 45° 角的方向伸展(花瓣状),原子核位于平面中心,x 轴和 y 轴为节面(电子密度为零的平面)。 -
d x z d_{xz} dxz
Electron density is concentrated in the x-z plane, extending 45° to the x and z axes (petal-like). The nucleus is at the plane center, with the x/z axes as nodal planes.
电子密度集中在 x-z 平面内,沿与 x 轴、z 轴成 45° 角的方向伸展(花瓣状),原子核位于平面中心,x 轴和 z 轴为节面。 -
d y z d_{yz} dyz
Electron density lies in the y-z plane, extending 45° to the y and z axes (petal-like). The nucleus is at the plane center, with the y/z axes as nodal planes.
电子密度分布在 y-z 平面内,沿与 y 轴、z 轴成 45° 角的方向伸展(花瓣状),原子核位于平面中心,y 轴和 z 轴为节面。 -
d x 2 − y 2 d_{x^2 - y^2} dx2−y2
Electron density extends directly along the positive/negative x and y axes (not 45°) in the x-y plane (petal-like), with the nucleus at the center. It is perpendicular to the d x y d_{xy} dxy orbital.
电子密度在 x-y 平面内,直接沿 x 轴正/负方向、y 轴正/负方向伸展(非 45° 角,花瓣状),原子核位于中心,与 d x y d_{xy} dxy 轨道相互垂直。 -
d z 2 d_{z^2} dz2
The most unique shape—electron density extends along the positive/negative z axes (forming two spindle-like regions) and has a ring-shaped (donut-like) distribution in the x-y plane. Unlike the other four d orbitals, it does not fit the typical “petal-like” description.
形态最特殊:电子密度沿 z 轴正/负方向伸展(形成两个纺锤状区域),同时在 x-y 平面内存在环形(甜甜圈状)电子密度分布。与其他四种 d 轨道不同,它不满足典型的“花瓣状”描述。
电子层排布式为什么是 s, p, d, f?
电子层排布式中的 s、p、d、f 轨道名称来源于光谱学研究,这些名称最初反映了光谱线的外观特征,并在量子力学的发展中被赋予了新的物理意义。
1. 电子亚层与原子轨道
电子亚层本质上是原子轨道。原子轨道是描述电子在原子中可能存在的空间区域的波函数。根据量子力学,电子以概率分布的形式存在于原子中,而非像经典物理学中那样在固定的轨道上运动。
2. 原子轨道的命名
原子轨道的命名源于光谱学研究。1904 年,日本物理学家长冈半太郎首次提出电子在原子内类似环绕轨道的运动概念。1926 年,量子力学的发展和薛定谔方程的提出解释了原子中电子的波动性质,引入了“轨道”(orbital)这一新概念。1932 年,美国化学家罗伯特·马利肯提出用“轨道”(orbital)取代“轨道”(orbit),以区分经典物理学中的轨道概念。原子轨道由主量子数 n n n、角量子数 l l l 和磁量子数 m m m 决定,分别对应电子的能量、角动量和空间取向。每个轨道最多可容纳两个电子,s、p、d、f 轨道分别对应角量子数 l = 0 , 1 , 2 , 3 l = 0, 1, 2, 3 l=0,1,2,3。
3. 光谱学的贡献
原子轨道的名称 s、p、d、f 起源于对碱金属光谱精细结构的研究。光谱学家根据光谱线的外观将它们分类为“sharp”(尖锐)、“principal”(主要)、“diffuse”(弥散)和“fundamental”(基本)。
- s 轨道:对应于“sharp”(尖锐)系列,角量子数 l = 0 l = 0 l=0。
- p 轨道:对应于“principal”(主要)系列,角量子数 l = 1 l = 1 l=1。
- d 轨道:对应于“diffuse”(弥散)系列,角量子数 l = 2 l = 2 l=2。
- f 轨道:对应于“fundamental”(基本)系列,角量子数 l = 3 l = 3 l=3。
这些术语后来被用来命名原子轨道,并在现代原子理论中得以保留。
4、延伸规则:f 之后的轨道命名
s、p、d、f 并非轨道的全部类型:当角量子数 l ≥ 4 l \geq 4 l≥4 时(对应主量子数 n ≥ 5 n \geq 5 n≥5),后续轨道按 字母顺序命名,但需跳过字母“E”(避免与其他科学符号混淆),即:
- l = 4 → g l = 4 \rightarrow g l=4→g 轨道
- l = 5 → h l = 5 \rightarrow h l=5→h 轨道
- l = 6 → i l = 6 \rightarrow i l=6→i 轨道
这些后续轨道(如 g、h)无早期光谱学含义,仅为按规则延续的标识,常见于重元素(如锕系、镧系元素)的电子排布中。
5、电子排布规则联
s、p、d、f 轨道的划分,是理解电子排布规律的关键:电子在原子内填充轨道需遵循三大规则,而轨道类型(s/p/d/f)直接决定填充顺序与稳定性:
- 能量最低原理:电子优先填充能量低的轨道,如 1 s < 2 s < 2 p < 3 s < 3 p < 4 s < 3 d 1s < 2s < 2p < 3s < 3p < 4s < 3d 1s<2s<2p<3s<3p<4s<3d(需注意 4s 能量低于 3d,故钾、钙先填 4s 再填 3d);
- 洪特规则:能量相同的轨道(如 3 个 p 轨道、5 个 d 轨道)中,电子先单独占据轨道(自旋平行),再成对填充;
- 泡利不相容原理:每个轨道最多容纳 2 个自旋相反的电子。
正是基于 s、p、d、f 轨道的能量差异与填充规则,我们才能理解各元素的电子排布式(如氧: 1 s 2 2 s 2 2 p 4 1s^2 2s^2 2p^4 1s22s22p4),进而解释原子的化学性质(如化合价、成键方式)。
via:
- The Origin of the s, p, d, f Orbital Labels by William B. Jensen
https://homepages.uc.edu/~jensenwb/reprints/137. s, p, d, f.pdf - Definition of sublevel-Chemistry Dictionary
https://www.chemicool.com/definition/sublevel.html - Energy Levels in Atoms: How Electrons Are Arranged
https://www.physicsclassroom.com/Chemistry-Tutorial/Modern-Atomic-Model/Energy-Levels - Atomic Orbitals Explained: s, p, d, and f Shapes
https://www.physicsclassroom.com/Chemistry-Tutorial/Modern-Atomic-Model/Orbitals - Schrödinger and the Wave-Mechanical Model of the Atom
https://www.physicsclassroom.com/Chemistry-Tutorial/Modern-Atomic-Model/Schrodinger-and-the-Wave-Mechanical-Model - How to Write Electron Configurations: Orbitals, Notation, and Rules
https://www.physicsclassroom.com/Chemistry-Tutorial/Modern-Atomic-Model/Electron-Configurations - The 4 Energy Sublevels Explained: A Simple s,p,d,f Guide-Eresources.blog
https://eresources.blog/4-energy-sublevels-explained-simple-s-p-d-f-guide - s,p,d,f Atomic Orbitals-Chemistry Steps
https://general.chemistrysteps.com/s-p-d-f-atomic-orbitals/ - Re: Where do the quantum numbers(n,l,m_l,m_s) and sublevels (s,p,d,f) come from
https://www.madsci.org/posts/archives/jan99/915460437.Sh.r.html - Why are the sublevels of an energy level called S,P,D,and F?-Factual Questions-Straight Dope Message Board
https://boards.straightdope.com/t/why-are-the-sublevels-of-an-energy-level-called-s-p-d-and-f/210331/6 - Why are orbitals called s,p,d,and f? • Physics Forums
https://www.physicsforums.com/threads/why-are-orbitals-called-s-p-d-and-f.53102/ - Orbitals SPDF.Why they named like that?-Chemistry Stack Exchange
https://chemistry.stackexchange.com/questions/55209/orbitals-spdf-why-they-named-like-that - About the meaning of the names of f orbitals-Chemistry Stack Exchange
https://chemistry.stackexchange.com/questions/21837/about-the-meaning-of-the-names-of-f-orbitals/21839 - what does the sublevel designations s,p,d,and f specify with respect to the atomic orbitals | Wyzant Ask An Expert
https://www.wyzant.com/resources/answers/3867/what-does-the-sublevel-designations-s-p-d-and-f-specify-with-respect-to-the-atomic-orbitals - 电子层排布式为什么是s,p,d,f? - 知乎 佐佐木一人 发布于 2021-04-07 09:11
https://www.zhihu.com/question/453270121 - 四面体场中dz²与dx²-y²轨道为什么简并? - 知乎 2023
https://www.zhihu.com/question/598429602 - Unlock Sodium’s Lewis Dot Structure: A Fast & Simple Guide Today!-Eresources.blog
https://eresources.blog/sodium-lewis-dot-structure-fast-guide





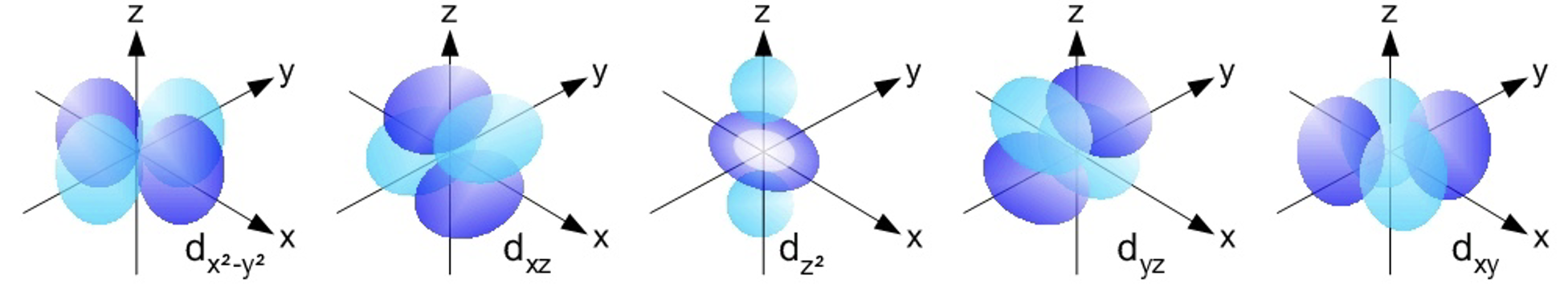
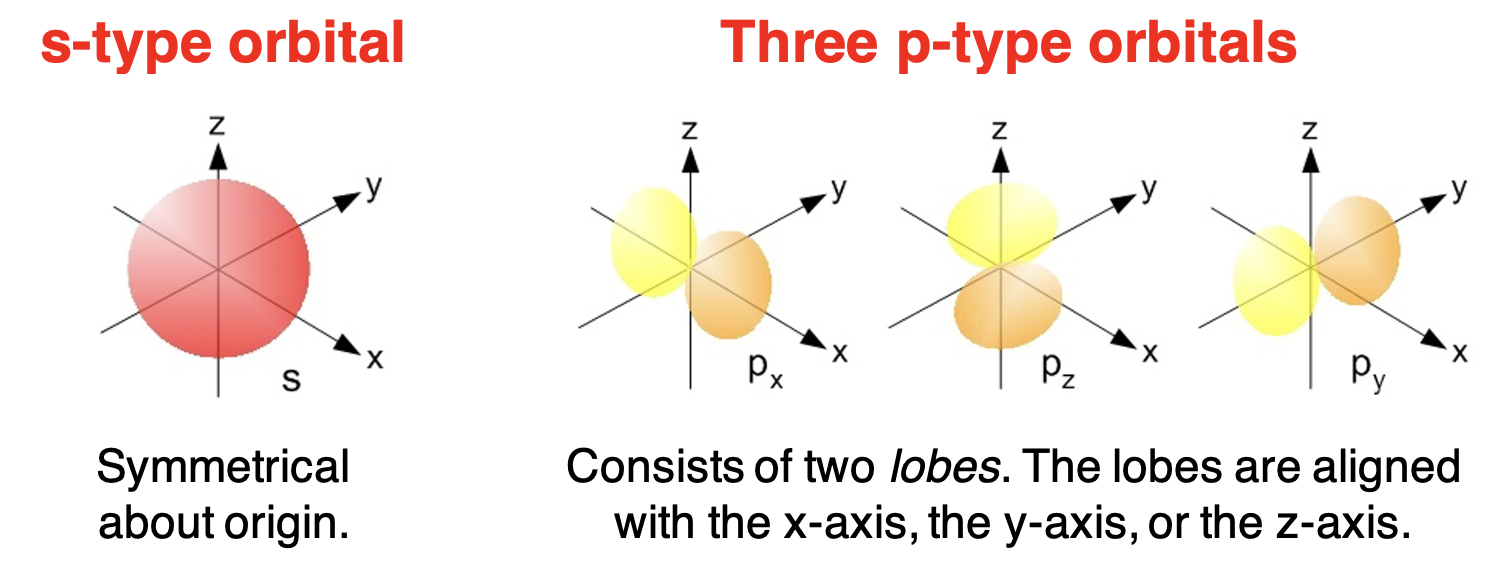
















 98
98

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








