注:本文为 “Electronic Structure of Atoms Rule Definition” 相关合辑。
英文引文,机翻未校。
如有内容异常,请看原文。
Hund’s Rule Definition and Examples
洪特规则的定义与示例
This entry was posted on March 16, 2023 by Anne Helmenstine (updated on March 20, 2023)
本文由安妮·赫尔门斯泰因于 2023 年 3 月 16 日发布(2023 年 3 月 20 日更新)

Hund’s rule states that electrons fill a suborbital singly and with the same spin before they form doubles with opposite spins.
洪特规则指出,电子在亚轨道中先单独填充,且具有相同的自旋,然后再形成具有相反自旋的成对电子。
In chemistry and atomic physics, Hund’s rule states that electrons fill a suborbital as singles before they start forming doubles and that all of the singles in the suborbital have the same spin. The rule gets it name for German physicist Friedrich Hund, who formulated it around 1927.
在化学和原子物理学中,洪特规则指出,电子在开始形成成对电子之前,先以单个电子的形式填充亚轨道,并且亚轨道中所有的单个电子都具有相同的自旋。该规则以德国物理学家弗里德里希·洪特的名字命名,他于 1927 年左右提出了这一规则。
What Is Hund’s Rule?
什么是洪特规则?
Hund’s rule describe the order in which electrons fill subshells and the spin quantum number of each electron:
洪特规则描述了电子填充亚壳层的顺序以及每个电子的自旋量子数:
- The orbitals of a subshell fill with single electrons before any subshells get double electrons (with antiparallel spin).
在任何亚壳层出现成对电子(具有反平行自旋)之前,亚壳层的轨道先填充单个电子。 - The single electrons in subshells have the same spin, so as to maximize total spin.
亚壳层中的单个电子具有相同的自旋,以最大化总自旋。
Basically, the lowest or most stable atomic state is the one that maximizes the total spin quantum number. Spin is either ½ or -½, so single electrons with the same value satisfies the rule. Another name for Hund’s rule is the “bus seat rule” because people choose separate seats on a bus before they start pairing up.
基本上,能量最低或最稳定的原子状态是总自旋量子数最大的状态。自旋要么是 ½ 要么是 -½,因此具有相同值的单个电子满足该规则。洪特规则的另一个名称是“公交车座位规则”,因为人们在公交车上会先选择分开的座位,然后才开始成对就坐。
Giving the single electrons in the orbitals the same spin minimizes electrostatic repulsion between electrons. While not entirely accurate, the classical example is that electrons orbiting an atom all in the same direction meet less often than if some went in one direction and some went in the opposite direction. Basically, single electrons in subshells have parallel spin because it is the most stable configuration.
让轨道中的单个电子具有相同的自旋可以最大限度地减少电子之间的静电排斥。虽然并不完全准确,但经典的例子是,围绕原子沿同一方向运动的电子相遇的次数,比一些沿一个方向运动而另一些沿相反方向运动的电子相遇的次数要少。基本上,亚壳层中的单个电子具有平行自旋,因为这是最稳定的构型。
Relationship to the Aufbau Principle and Pauli Exclusion Principle
与泡利不相容原理和能量最低原理的关系
The Aufbau principle and Hund’s rule both describe how electrons fill orbitals, but the Aufbau principle explains the order in which electrons fill orbitals, while Hund’s rule describes how, exactly, electrons fill those orbitals.
能量最低原理和洪特规则都描述了电子如何填充轨道,但能量最低原理解释了电子填充轨道的顺序,而洪特规则则具体描述了电子如何填充这些轨道。
The Aufbau principle states that electrons fill the subshells of the lowest energy orbital before moving on to higher energy subshells. For example, electrons fill the 1s subshell before any electrons enter the 2s subshell. This way, electrons achieve the most stable electron configuration.
能量最低原理指出,电子先填充能量最低的轨道亚壳层,然后再填充能量较高的亚壳层。例如,电子先填充 1s 亚壳层,然后才会有电子进入 2s 亚壳层。通过这种方式,电子达到最稳定的电子构型。
Hund’s rule describes the way these electrons fill the lowest energy subshell, where electrons half-fill subshells with electrons having the same spin before that subshell gets two electrons. Those two electrons have opposite spin values due to the Pauli exclusion principle.
洪特规则描述了这些电子填充最低能量亚壳层的方式,即在亚壳层获得两个电子之前,电子先以相同的自旋半填充亚壳层。由于泡利不相容原理,这两个电子具有相反的自旋值。
The Pauli exclusion principle states that a maximum of two electrons can occupy an orbital and they have opposite or antiparallel spin values because no two electrons in an atom have the exact same quantum numbers.
泡利不相容原理指出,一个轨道最多只能容纳两个电子,且它们具有相反或反平行的自旋值,因为原子中没有两个电子具有完全相同的量子数。
Aufbau Rule Examples
洪特规则示例
Nitrogen Atom
氮原子
The electron configuration of a nitrogen atom (Z=7) is 1s² 2s² 2p³. Using Hund’s rule, show how electrons fill the subshells.
氮原子(Z=7)的电子构型为 1s² 2s² 2p³。根据洪特规则,电子填充亚壳层的方式如下。
Here, the 1s and 2s subshells are filled. The 2p subshell is only half-filled. So, the electrons in the 1s and 2s subshells are pairs and antiparallel, while the 3 electrons in the 2p subshell are separate from each other and have the same spin:
在这里,1s 和 2s 亚壳层已填满。2p 亚壳层仅半满。因此,1s 和 2s 亚壳层中的电子是成对的且自旋反平行,而 2p 亚壳层中的 3 个电子彼此分离且具有相同的自旋:

Oxygen Atom
氧原子
Oxygen follows nitrogen on the periodic table (Z=8). Its electron configuration is 1s² 2s² 2p⁴. The filling of the 1s and and 2s subshells is the same as for nitrogen, but there is an additional electron in the 2p subshell. First, fill each subshell with a single electron. Add the additional electron to make a pair and make it antiparallel to the first electron:
在元素周期表中,氧位于氮之后(Z=8)。其电子构型为 1s² 2s² 2p⁴。1s 和 2s 亚壳层的填充方式与氮相同,但 2p 亚壳层多了一个电子。首先,每个亚壳层填充一个电子。再添加一个电子形成一对,且与第一个电子自旋反平行:

Importance of Hund’s Rule
洪特规则的重要性
Hund’s rule is important because it shows how electrons organize into subshells. This identifies the valence electrons (the unpaired ones), which are the electrons that participate in chemical reactions and account for much of an atom’s chemical properties. For example, the electron configuration reflects an atom’s stability. An atom with only one unpaired electron is highly reactive, while one with no unpaired electrons is stable. The valence shell also indicates the magnetic properties of an atom. If there are unpaired electrons, the atom is paramagnetic and attracted to a magnetic field. If all of the electrons are paired, the atom is diamagnetic and is weakly repelled by a magnetic field.
洪特规则很重要,因为它展示了电子如何在亚壳层中排列。这有助于确定价电子(未成对的电子),价电子是参与化学反应的电子,并在很大程度上决定了原子的化学性质。例如,电子构型反映了原子的稳定性。只有一个未成对电子的原子具有高反应性,而没有未成对电子的原子则很稳定。价层还能表明原子的磁性。如果存在未成对电子,原子具有顺磁性,会被磁场吸引。如果所有电子都成对,则原子具有抗磁性,会被磁场微弱排斥。
References
参考文献
- Cottingham, W. N.; Greenwood, D. A. (1986). “Chapter 5: Ground state properties of nuclei: the shell model”. An Introduction to Nuclear Physics. Cambridge University Press. ISBN 0-521-31960-9.
Cottingham, W. N.;Greenwood, D. A.(1986)。“第 5 章:原子核的基态性质:壳层模型”。《核物理学导论》。剑桥大学出版社。ISBN 0-521-31960-9。 - Engel, T.; Reid, P. (2006). Physical Chemistry. Pearson Benjamin-Cummings. ISBN 080533842X.
Engel, T.;Reid, P.(2006)。《物理化学》。培生·本杰明-卡明斯出版社。ISBN 080533842X。 - Goudsmit, S. A.; Richards, Paul I. (1964). “The Order of Electron Shells in Ionized Atoms”. Proc. Natl. Acad. Sci. 51 (4): 664–671. doi:10.1073/pnas.51.4.664
Goudsmit, S. A.;Richards, Paul I.(1964)。“电离原子中电子壳层的顺序”。《美国国家科学院院刊》。51(4):664-671。doi:10.1073/pnas.51.4.664 - Klechkovskii, V.M. (1962). “Justification of the Rule for Successive Filling of (n+l) Groups“. Journal of Experimental and Theoretical Physics. 14 (2): 334.
Klechkovskii, V.M.(1962)。“(n + l) 组依次填充规则的证明”。《实验与理论物理杂志》。14(2):334。 - Miessler, G.L.; Tarr, D.A. (1999). Inorganic Chemistry (2nd ed.). Prentice-Hall. ISBN 0138418918.
Miessler, G.L.;Tarr, D.A.(1999)。《无机化学》(第 2 版)。普伦蒂斯-霍尔出版社。ISBN 0138418918。
Electron Configurations
电子构型
Electron configurations tell us the number and location of electrons in an atom or an ion. Let’s first write a simple electron configuration and see what each letter and number indicate. For example, hydrogen has an electron configuration of 1s¹. So, what do the letter, and the numbers tell us?
电子构型告诉我们原子或离子中电子的数量和位置。让我们先写出一个简单的电子构型,看看每个字母和数字代表什么。例如,氢的电子构型是1s¹。那么,这些字母和数字告诉我们什么呢?
The first number in front of the “s” indicates the main energy level, and this is the principal quantum number, n. Remember, what we discussed about the Bohr model of the hydrogen atom. There are orbits with fixed radii each associated with discrete energy, and this is described by the principal quantum number n.
“s”前面的第一个数字表示主能级,这就是主量子数 n。还记得我们讨论过的氢原子的玻尔模型)。氢原子有固定半径的轨道,每个轨道都与离散的能量相关联,这由主量子数 n 来描述。

Next, we have the letter “s”, and this is the type of the orbital that the electron(s) is, and this is the energy sublevel because, for example, s and p orbitals in the same level have different energies. The number 1 given in the exponent tells us how many electrons we have in the s orbital that is in the first energy level:
接下来,我们看到字母“s”,它表示电子所在的轨道类型,也就是能级亚层,因为例如,同一能级中的 s 轨道和 p 轨道具有不同的能量。指数中的数字 1 告诉我们在第一能级的 s 轨道中有多少个电子:

So, the number before each letter (except for the d and f orbitals that we will get to) can easily be determined based on the period of the periodic table where the element is located.
因此,每个字母前面的数字(我们后面会讲到的 d 和 f 轨道除外)可以根据元素在元素周期表中的周期轻松确定。
Let’s confirm this by looking at the electron configuration of fluorine: 1s²2s²2p⁵.
让我们通过氟的电子构型来确认这一点:1s²2s²2p⁵。
The 1s² means there are 2 electrons in the s orbital that is in the first row. Next, we have the orbitals in the second energy level because fluorine is in the second row of the periodic table. In this level, there are 2 electrons in an s orbital, and 5 electrons in the p orbital:
1s² 表示第一周期的 s 轨道中有 2 个电子。接下来,我们看第二能级的轨道,因为氟位于元素周期表的第二周期。在这个能级中,s 轨道有 2 个电子,p 轨道有 5 个电子:

The total number of electrons must be equal to the atomic number of the element because remember, the atomic number shows the number of protons which, for a neutral atom, is equal to the number of electrons. The atomic number of fluorine is 9, and if we sum all the electrons in the configuration, we should get that number (2 + 2 + 5 = 9).
电子的总数必须等于该元素的原子序数,因为请记住,原子序数表示质子数,对于中性原子来说,质子数等于电子数。氟的原子序数是 9,如果我们把构型中的所有电子数相加,应该得到这个数字(2 + 2 + 5 = 9)。
To summarize, we can see that the electron configuration shows first the principal energy level (n value), then the energy sublevel (l value – this is the type of the orbital i.e., s, p, d or f), and the number of electrons in this sublevel given as a superscript.
总而言之,电子构型首先显示主能级(n 值),然后是能级亚层(l 值——即轨道类型,如 s、p、d 或 f),右上角的上标表示该亚层中的电子数。

Orbital Characteristics and Orbital Diagrams
轨道特征和轨道图
Now, a few important things about the orbitals and their electron capacity. First, remember that each orbital, whether it is s, p, d, or f can accommodate two electrons at most. So, you can never write things like 2s³ or 3p⁷, etc. The reason for 3p⁷ being wrong is that there are only 3 p orbitals in each energy level and because each can only take two electrons, the maximum number of electrons in the p sublevel is six. The orbitals are often shown as squares with electrons in them represented as arrows. These are called orbital diagrams:
现在,关于轨道及其电子容纳能力有几点重要内容需要了解。首先,请记住,每个轨道,无论是 s、p、d 还是 f 轨道,最多只能容纳两个电子。所以,你绝不能写出像2s³或3p⁷这样的式子。3p⁷是错误的,因为每个能级中只有 3 个 p 轨道,且每个轨道只能容纳两个电子,所以 p 亚层最多能容纳 6 个电子。轨道通常用方框表示,其中的电子用箭头表示。这些被称为轨道图:

If there are two electrons in the orbitals, the arrows must be shown in opposite directions which indicates that they must have opposite spins. This is according to the Pauli exclusion principle which states that no two electrons in an atom can have the same four quantum numbers. So, if the electrons are in the same orbital, they must have the same n, l, mₗ, and therefore, the only one that can be different is the mₛ which is the spin of the electron shown by the position of the arrow.
如果轨道中有两个电子,箭头必须方向相反,这表明它们必须具有相反的自旋。这是根据泡利不相容原理,该原理指出一个原子中没有两个电子可以具有相同的四个量子数。因此,如果电子在同一个轨道中,它们的 n、l、mₗ 必须相同,那么唯一可以不同的就是 mₛ(即电子的自旋),这通过箭头的方向来表示。
Next, remember the number and capacity of s, p, d, and f energy sublevels. There can only be 1 s orbital in a given energy level, 3 p orbitals, 5 d orbitals, and 7 f orbitals. And because each orbital can only take a maximum of two electrons, there can only be a maximum of two electrons in any s sublevel, 6 electrons in the p subshell, 10 in the d, and 14 in the f sublevel.
接下来,记住 s、p、d 和 f 能级亚层的数量和容量。在一个给定的能级中,只能有1 个 s 轨道、3 个 p 轨道、5 个 d 轨道和7 个 f 轨道。由于每个轨道最多只能容纳两个电子,因此任何s 亚层最多只能有2 个电子,p 亚层最多有6 个电子,d 亚层最多有10 个电子,f 亚层最多有14 个电子。

The next question we want to address is how to easily recognize what orbitals a given element has. For this, try to memorize this orbital map of the periodic table:
接下来我们要解决的问题是如何轻松识别特定元素具有哪些轨道。为此,请尝试记住元素周期表的这个轨道分布图:

Rules of the Aufbau Principle
能量最低原理规则
Aufbau (German aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. An easy example illustrating the idea behind the aufbau principle would be pouring water into a tube structure with chambers at different height levels:
Aufbau(德语“aufbauen”,意为“构建”)原理告诉我们,电子按照能量递增的顺序填充轨道。一个简单的例子可以说明能量最低原理背后的思想:往一个有不同高度腔室的管状结构中倒水。

It is clear that the water is going to fill first the chambers in lower levels before starting to go into the ones at a higher level. We can explain this in terms of gravity, and potential energy as the chambers in lower levels have lower energy levels and therefore, the water in them would have lower potential energy which means higher stability. Remember, any system always tends to move towards a lower energy state.
显然,水会先填满较低位置的腔室,然后才开始进入较高位置的腔室。我们可以用重力和势能来解释这一点,因为较低位置的腔室能量较低,因此其中的水势能较低,也就意味着更稳定。请记住,任何系统都倾向于朝着能量更低的状态发展。
The energy levels of the orbitals can be shown as follows:
轨道的能量水平可以表示如下:

Notice that within the same principal level, orbitals with a lower value of l have lower energy (E) and therefore, are filled first. So, for a given value of n:
注意,在同一个主能级中,l 值较小的轨道能量(E)较低,因此会先被填充。所以,对于给定的 n 值:
E (s orbital) < E (p orbital) < E (d orbital) < E (f orbital)
Now, in the periodic table, it may look a little different as the orbitals with lower energy levels are generally in the higher-standing rows. This is because remember, the rows (periods) of the periodic table indicate the principal quantum number (n), which is the energy level, and they increase as we go down the periodic table:
现在,在元素周期表中,情况可能看起来略有不同,因为能量较低的轨道通常位于位置更靠上的周期中。这是因为请记住,元素周期表的行(周期)表示主量子数(n),即能级,并且随着我们沿着周期表向下移动,主量子数会增大:

Considering this, and bringing back the analogy of water filling the chambers, you can visualize this pattern for filling the orbitals:
考虑到这一点,再结合水填充腔室的类比,你可以想象出轨道填充的模式:

The water (electrons) fills first the s level, then the p, then the d, and the f subshells of the given energy level. There are some deviations when it comes to the d and f orbitals, and we will discuss that later in the article.
水(电子)会先填充给定能级的 s 亚层,然后是 p 亚层,接着是 d 亚层,最后是 f 亚层。对于 d 和 f 轨道,存在一些填充顺序的偏差,我们将在本文后面讨论这一点。
Let’s now do some more examples. First is the hydrogen, and it is in the first row, therefore, the only electron it has is going to be in the 1s level, and the electron configuration will be 1s¹:
现在我们再举一些例子。首先是氢,它位于第一周期,因此,它仅有的一个电子会在 1s 能级,电子构型为 1s¹:

For helium (He), it will be 1s² because it has two electrons, and the s orbital can still accommodate one more electron.
对于氦(He),其电子构型为 1s²,因为它有两个电子,而 s 轨道还能再容纳一个电子。

Next, we have lithium. Now, it is in the second row, but before getting there, we must use the 1s orbital. Remember, water couldn’t jump up without filling the lower levels. So, lithium has 3 electrons (atomic number), and therefore, the electron configuration will be 1s²2s¹.
接下来是锂。锂位于第二周期,但在填充第二周期的轨道之前,我们必须先填满 1s 轨道。记住,就像水不会不填满低处就跳到高处一样,电子也是如此。锂有 3 个电子(原子序数为 3),因此其电子构型为 1s²2s¹。

Consequently, beryllium (Be) will have one more electron with a configuration of 1s²2s².
因此,铍(Be)会再多一个电子,其构型为 1s²2s²。

Next, we have boron (B), and it is in the region of p orbitals. Therefore, everything we had for Be is still going to be in place, and the one additional electron will go to the 2p orbital:
接下来是硼(B),它位于 p 轨道区域。因此,铍的电子构型部分保持不变,多出来的一个电子会进入 2p 轨道:

Now, carbon © is going to add one more electron in the p sublevel thus having 1s²2s²2p² electron configuration. What is important is that this electron goes to the next (empty) p orbital rather than fitting in with the other electron:
现在,碳(C)会在 p 亚层再增加一个电子,因此其电子构型为 1s²2s²2p²。重要的是,这个电子会进入**下一个(空的)**p 轨道,而不是与另一个电子挤在同一个轨道中:

This is the Hund’s rule, which states that electrons will fill all the degenerate orbitals (equal in energy) with parallel spins (both arrows up or down) first before pairing up in one orbital. We can also formulate it as the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons within the same energy sublevel.
这就是洪特规则,该规则指出,电子会先以平行自旋(箭头都向上或都向下)填充所有简并轨道(能量相同),然后再在同一个轨道中配对。我们也可以将其表述为:原子的最低能量构型是在同一能级亚层中具有最多未成对电子的构型。
Hund’s rule is another demonstration of the same principle which is the tendency to adopt the lowest energy state possible. There is a stronger repulsive interaction between two electrons in the same orbital compared to when they occupy separate orbitals of equal energy.
洪特规则是同一原理的另一种体现,即倾向于采取可能的最低能量状态。与两个电子占据能量相同的不同轨道相比,同一轨道中的两个电子之间的排斥作用更强。
So, as expected, the next element, nitrogen (N) will place the additional electron in the 3rd p orbital which is empty in carbon.
因此,正如预期的那样,下一个元素氮(N)会将额外的电子放在碳中为空的第三个 p 轨道上。

Following the same principle, we get to neon (Ne) which has a complete filling of the n = 2 principal level.
按照同样的原理,我们来到氖(Ne),它的 n = 2 主能级被完全填满。

Now, there is something special about (completely) filled orbitals. This makes the electrons very low in energy meaning they are stable and cannot normally lower their energy more by participating in chemical reactions. Therefore, Ne and all the other noble gases below it are the most chemically stable and thus unreactive (inert) family in the periodic table.
现在,(完全)填满的轨道有其特殊之处。这使得电子能量非常低,意味着它们很稳定,通常不能通过参与化学反应来进一步降低能量。因此,氖以及周期表中位于其下方的所有其他稀有气体是化学性质最稳定且不活泼(惰性) 的一族元素。
The first element in period 3 is sodium (Na), and we are going to write the electron configuration of Ne with an additional electron in the 3s sublevel:
第三周期的第一个元素是钠(Na),我们可以写出氖的电子构型,再加上 3s 亚层的一个额外电子:

Writing an Electron Configuration for an Element from Its Position in the Periodic Table
根据元素在周期表中的位置书写电子构型
At some point, when you start noticing the pattern of filling the orbitals and their mapping on the periodic table, it may be easier to write the electron configuration simply based on the position of the element. So, instead of starting from 1s²…, we write the electron configuration of the last noble gas that comes before the element and then add the remaining electrons from the corresponding n level. For example, let’s say we want to write an electron configuration for sulfur (S). First, you need to write the inner electron configuration, that is the configuration of the noble gas that precedes it in the periodic table. In this case, it is Ne, and since it is the last element in the second row, the last filled sublevel would be 2p⁶, and therefore, the electron configuration of the noble gas will be 1s²2s²2p⁶. Now, to this, we add 2 s electrons and four p electrons since S is the fourth element in the p orbital area:
在某个时候,当你开始注意到轨道的填充模式以及它们在周期表上的对应关系时,仅仅根据元素的位置来书写电子构型可能会更容易。所以,我们不用从 1s²…开始写,而是写出该元素之前的最后一个稀有气体的电子构型,然后再加上相应 n 能级的剩余电子。例如,假设我们要写出硫(S) 的电子构型。首先,你需要写出内层电子构型,即该元素在周期表中前面的稀有气体的构型。在这种情况下,是氖(Ne),由于它是第二周期的最后一个元素,最后填满的亚层是 2p⁶,因此该稀有气体的电子构型为 1s²2s²2p⁶。现在,在此基础上,我们再加上 2 个 s 电子和 4 个 p 电子,因为硫是 p 轨道区域的第四个元素:

Therefore, the electron configuration will be 1s²2s²2p⁶3s²3p⁴.
因此,硫的电子构型为 1s²2s²2p⁶3s²3p⁴。
Condensed electron configurations
简化电子构型
In condensed electron configurations, we show the symbol of the noble gas that precedes the element in square brackets, and then add the remaining electrons just as we have been doing so far. So, for sulfur, it will be S: [Ne]3s²3p⁴.
在简化电子构型中,我们把元素前面的稀有气体符号放在方括号中,然后像之前那样加上剩余的电子。所以,硫的简化电子构型为 S:[Ne]3s²3p⁴。
Another example is the electron configuration of bromine (Br). Since it is in the 4th row, the inner configuration would be that of argon (Ar), and after that, we add the electrons as we did for the d elements that we have discussed. Two electrons will go into the 4s orbital, 10 to the 3d orbitals, and 5 into the 4p sublevel – Br: [Ar]4s²3d¹⁰4p⁵.
另一个例子是溴(Br)的电子构型。由于它位于第四周期,内层构型是氩(Ar) 的构型,之后,我们按照讨论过的 d 区元素的方式添加电子。2 个电子进入 4s 轨道,10 个电子进入 3d 轨道,5 个电子进入 4p 亚层——Br:[Ar]4s²3d¹⁰4p⁵。
Electron Configuration of d Elements – the Transition Series
d 区元素的电子构型——过渡系列
There is one exception to keep in mind for the electron configuration of transition metals. That is the (n +1)s orbitals always fill before the nd orbitals.
过渡金属的电子构型有一个例外需要记住。即 (n + 1)s 轨道总是在 nd 轨道之前填充。
For example, the 4s level fills before the 3d level, and therefore, the electron configuration of titanium (Ti) is 1s²2s²2p⁶3s²3p⁶4s²3d² or, the condensed configuration which will be [Ar]4s²3d².
例如,4s 能级在 3d 能级之前填充,因此钛(Ti)的电子构型为 1s²2s²2p⁶3s²3p⁶4s²3d²,其简化构型为 [Ar]4s²3d²。

As we get to higher values of n, other variations in the filling pattern occur because the energy sublevels become very close together (recall the figure on the energy levels above).
当我们涉及到更高的 n 值时,填充模式会出现其他变化,因为能级亚层变得非常接近(回想一下上面关于能级的图)。
Stability of Half-Filled and Filled Sublevels
半满和全满亚层的稳定性
We mentioned that noble gases are characterized by great stability because of their completely filled orbitals. Now, similar to this, orbitals tend to get lower energy levels (high stability) when they are half-filled. For example, following what we have learned so far, we may expect the following electron configuration for chromium (Cr): [Ar]4s²3d⁴.
我们提到过,稀有气体因其完全填满的轨道而具有很高的稳定性。类似地,当轨道半满时,它们往往具有较低的能级(高稳定性)。例如,根据我们到目前为止所学的知识,我们可能会认为铬(Cr)的电子构型为:[Ar]4s²3d⁴。
However, the electron configuration of Cr is [Ar]4s¹3d⁵, and the reason for this is that the d orbital gets a half-filled configuration (remember
d
d
d orbitals can have a maximum of 10 electrons). We can think of this as the electron jumping from the 4s level to the 3d level and compensating this energy uphill by a stabilization associated with half-filled orbitals:
然而,铬的电子构型是 [Ar]4s¹3d⁵,原因是 d 轨道获得了半满构型(记住 d 轨道最多可容纳 10 个电子)。我们可以这样理解:一个电子从 4s 能级跳到 3d 能级,而半满轨道带来的稳定性弥补了这一能量上的提升:

Of course, in reality, it is not as though the electrons fill one by one, and then one of them jumps from 4s to 3d level. However, this is a visual representation to help better understand this behavior in the electron configuration of transition elements.
当然,实际上,并不是电子先一个一个填充,然后其中一个从 4s 跳到 3d 能级。不过,这是一种直观的表示,有助于更好地理解过渡元素电子构型中的这种行为。
Let’s also discuss the electron configuration of copper (Cu) which stands before zinc (Zn), and one may expect it to have 9 electrons in the d sublevel. However, the correct electron configuration of Cu is [Ar]4s¹3d¹⁰ as this allows to attain filled d orbitals and a half-filled s orbital which is lower in energy (more stable) than the expected [Ar]4s²3d⁹ configuration.
我们还来讨论一下铜(Cu) 的电子构型,它位于锌(Zn)之前,人们可能会认为它的 d 亚层有 9 个电子。然而,Cu 的正确电子构型是 [Ar]4s¹3d¹⁰,因为这样可以获得全满的 d 轨道和半满的 s 轨道,这比预期的 [Ar]4s²3d⁹ 构型能量更低(更稳定)。

Inner Transition Series (f Elements)
内过渡系列(f 区元素)
Starting from period 6, we have the inner transition elements which contain f orbitals. The first thing you need to remember here is that there are seven f orbitals because l = 3, so the possible mₗ values are −3, −2, −1, 0, +1, +2, and +3. Each orbital can accommodate two electrons and therefore, a total of 14 electrons can fill the given f sublevel.
从第六周期开始,我们遇到了包含 f 轨道的内过渡元素。这里你需要记住的第一件事是,有7 个 f 轨道,因为 l = 3,所以可能的 mₗ 值为 -3、-2、-1、0、+1、+2 和 +3。每个轨道可以容纳两个电子,因此,给定的 f 亚层总共可以填充 14 个电子。
Although the first element in this region is lanthanum (La), the filling of f orbitals starts from cerium (Ce), and the elements together with it in Period 6 are called lanthanides (or rare earth elements).
尽管该区域的第一个元素是镧(La),但 f 轨道的填充从铈(Ce)开始,第六周期中与铈一起的这些元素被称为镧系元素(或稀土元素)。

Notice the general filling order is 6s → 4f → 5d, however, ns goes first, then only the first of (n − 1)d, all (n − 2)f, and after that remainder of the (n − 1)d, and np. Therefore, the electron configuration of La is [Xe]6s²5d¹ and not [Xe]6s²4f¹ as the first d electron goes first.
注意,一般的填充顺序是 6s → 4f → 5d,然而,ns 先填充,然后是仅(n - 1)d 的第一个轨道,所有(n - 2)f 轨道,之后是(n - 1)d 的剩余轨道和 np 轨道**。因此,镧的电子构型是 [Xe]6s²5d¹,而不是 [Xe]6s²4f¹,因为第一个 d 电子先填充。
The inner transition series in Period 7 start after actinium (Ac; Z = 89) and are called the actinides.
第七周期的内过渡系列从锕(Ac;Z = 89)之后开始,被称为锕系元素。
Hopefully, you won’t get tested on remembering all these exceptions as it should be the principle that is more important, but in any case, use the [ptable.com](https://ptable.com/## Electrons/Configuration) website to practice filling electron configurations. Just be sure to click the “Electrons” tab in the upper area.
希望你不会被考查记住所有这些例外情况,因为原理更为重要,但无论如何,可以使用 [ptable.com](https://ptable.com/## Electrons/Configuration) 网站来练习填充电子构型。只需确保点击上方区域的“Electrons”选项卡。
Valence Electrons and Electron Configurations
价电子与电子构型
Valence electrons are the ones farthest away from the nucleus and therefore, we find them in the outermost two orbitals. For example, Li (1s²2s¹) has one valence electron, and it is in the 2s orbital. Chlorine (1s²2s²2p⁶3s²3p⁵) has seven valence electrons in the 3s and 3p sublevels. If you ever forget, remember that you can easily double-check this based on the group number. The group number indicates the number of valence electrons in the atom.
价电子是离原子核最远的电子,因此,我们可以在最外层的两个轨道中找到它们。例如,Li(1s²2s¹)有一个价电子,位于 2s 轨道。氯(1s²2s²2p⁶3s²3p⁵)在 3s 和 3p 亚层有 7 个价电子。如果你忘记了,可以根据族序数轻松复查。族序数表示原子中价电子的数量。
Electron Configuration of Ions
离子的电子构型
The first thing you need to remember here is that cations are formed by losing an electron(s), and anions are formed by gaining an electron(s).
这里你需要记住的第一件事是,阳离子通过失去电子形成,阴离子通过获得电子形成。
The charge of the ion is a result of an imbalance between the number of protons and electrons. If it is a cation, then the positive charge indicates how many more protons it has compared to the number of electrons. For anions, the charge tells how many extra electrons there are compared to the number of protons.
离子的电荷是质子数和电子数不平衡的结果。如果是阳离子,则正电荷表示它比电子数多的质子数。对于阴离子,电荷表示比质子数多的电子数。
Recall this pattern for the formation of anions and cations: Metals tend to lose electron(s) and become cations (positively charged ions).
回想一下阴离子和阳离子形成的模式:金属倾向于失去电子,成为阳离子(带正电的离子)。

Nonmetals tend to gain an electron(s) and become anions (negatively charged ions).
非金属倾向于获得电子,成为阴离子(带负电的离子)。

Notice that the number of protons is not changed, and the ions are charged because, unlike atoms, their number of protons and electrons is not equal.
注意,质子数没有改变,离子带电是因为与原子不同,它们的质子数和电子数不相等。
Electron Configuration of Main Group Cations
主族阳离子的电子构型
Now, how do we determine the electron configuration of an ion? If it is a +1 charged cation, that means the atom has lost one electron. This electron is going to be from the outermost valence shell as these are the electrons farthest away from the nucleus and thus not as strongly attracted to it.
现在,我们如何确定离子的电子构型呢?如果是 +1 价阳离子,这意味着原子失去了一个电子。这个电子来自最外层的价层,因为这些电子离原子核最远,因此受到的吸引力不强。
Let’s see an example with Na. It is in the first group, so it loses one electron to become Na⁺. The electron configuration of sodium is 1s²2s²2p⁶3s¹, and the electron is removed from the energy level with the greatest n value – 3s. Therefore, the electron configuration of the Na⁺ ion will be 1s²2s²2p⁶:
让我们看一个钠的例子。它位于第Ⅰ族,因此失去一个电子成为Na⁺。钠的电子构型是 1s²2s²2p⁶3s¹,电子从 n 值最大的能级——3s 中移除。因此,Na⁺ 离子的电子构型为1s²2s²2p⁶:

Notice that the ion has a configuration with a complete shell of p orbitals which is characteristic of noble gases. In fact, this is the electron configuration of Ne, and we say that Na⁺ and Ne are isoelectronic (same electronic structure). The reason for this is that, remember, noble gases are very stable because of the low energy level of complete orbitals.
注意,该离子具有全满的 p 轨道壳层,这是稀有气体的特征。事实上,这是氖的电子构型,我们说 Na⁺ 和 Ne 是等电子体(具有相同的电子结构)。原因是,如前所述,稀有气体由于全满轨道的低能级而非常稳定。
This pattern explains why the metals in the first group become +1, the ones in the second group become +2, and Al, for example, becomes +3. It takes removing one electron from a metal in the first group to obtain the electron configuration of the previous noble gas, it takes two for the group two metals, etc.
这种模式解释了为什么第Ⅰ族的金属形成 +1 价,第Ⅱ族的金属形成 +2 价,例如铝形成**+3 价**。第Ⅰ族的金属失去一个电子即可获得前一个稀有气体的电子构型,第Ⅱ族的金属需要失去两个电子,依此类推。
Na (1s²2s²2p⁶3s¹) ⟶ e⁻ + Na⁺ ([He]2s²2p⁶) [isoelectronic with Ne ([He] 2s²2p⁶)]
Na (1s²2s²2p⁶3s¹) ⟶ e⁻ + Na⁺ ([He]2s²2p⁶) [与 Ne 等电子 ([He] 2s²2p⁶)]
Electron Configuration of Anions
阴离子的电子构型
Anions are formed when the atom gains as many electrons as necessary to attain the electron configuration of the next noble gas in the periodic table. For example, oxygen (O) is in group 6, and therefore, it will need two electrons to attain the electron configuration of Ne:
当原子获得所需数量的电子以达到周期表中下一个稀有气体的电子构型时,就形成了阴离子。例如,氧(O) 位于第Ⅵ族,因此,它需要两个电子才能达到 Ne 的电子构型:

Notice again that the two electrons go to the outermost valence orbital. Oxygen has 6 valence electrons – those in the 2s and 2p orbitals, however, since p sublevels are higher in energy, and they are the only ones capable of accepting additional electrons, the two electrons go to the 2p orbitals. The electron configuration of the oxide ion (O²⁻) is therefore, 1s²2s²2p⁶.
再次注意,这两个电子进入最外层的价轨道。氧有 6 个价电子——位于 2s 和 2p 轨道中,然而,由于 p 亚层能量更高,且是唯一能够接受额外电子的轨道,所以这两个电子进入 2p 轨道。因此,氧离子(O²⁻)的电子构型为1s²2s²2p⁶。
O (1s²2s²2p⁴) + 2e⁻ ⟶ O²⁻ ([He]2s²2p⁶) [isoelectronic with Ne ([He] 2s²2p⁶)]
O (1s²2s²2p⁴) + 2e⁻ ⟶ O²⁻ ([He]2s²2p⁶) [与 Ne 等电子 ([He] 2s²2p⁶)]
Another common type of monoatomic anions are the halides. Halogens are in group 7, and therefore, they only need one electron to attain the electron configuration of the noble gas following them in the periodic table. For example, bromine takes one electron and becomes isoelectronic to Kr:
另一种常见的单原子阴离子是卤化物。卤素位于第Ⅶ族,因此,它们只需要一个电子就能达到周期表中紧随其后的稀有气体的电子构型。例如,溴获得一个电子后与 Kr 成为等电子体:
Br ([Ar] 4s²3d¹⁰4p⁵) + e⁻ ⟶ Br⁻ ([Ar] 4s²3d¹⁰4p⁶) [isoelectronic with Kr ([Ar] 4s²3d¹⁰4p⁶)]
Br ([Ar] 4s²3d¹⁰4p⁵) + e⁻ ⟶ Br⁻ ([Ar] 4s²3d¹⁰4p⁶) [与 Kr 等电子 ([Ar] 4s²3d¹⁰4p⁶)]
Electron Configuration of Transition Metal Cations
过渡金属阳离子的电子构型
In contrast to main-group ions, transition metal ions do not usually attain a noble gas configuration. This is because the ns level is the outermost level, and the (n-1)d is considered an inner level therefore, it will take too much energy to remove those electrons and achieve a noble gas configuration. Therefore, the cation of a transition metal is formed by removing first the electrons from the ns (highest principal quantum number) orbital and then from the (n -1)d orbitals.
与主族离子不同,过渡金属离子通常不会达到稀有气体构型。这是因为 ns 能级是最外层能级,而 (n-1)d 被视为内层能级,因此移除这些电子以达到稀有气体构型需要太多能量。因此,过渡金属阳离子通过先从 ns(最高主量子数)轨道移除电子,然后从 (n -1)d 轨道移除电子而形成。
For example, the electron configuration of Zn is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰ or [Ar]4s²3d¹⁰, and it loses the two electrons from the 4s orbital to become Zn²⁺ [Ar]3d¹⁰. This is “not a bad” electron configuration considering the filled d orbitals.
例如,Zn 的电子构型是 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰ 或 [Ar]4s²3d¹⁰,它从 4s 轨道失去两个电子形成 Zn²⁺ [Ar]3d¹⁰。考虑到全满的 d 轨道,这是一种“不错的”电子构型。

Excited and Ground State Electron Configurations
激发态和基态电子构型
Remember, when discussing the Bohr model of the hydrogen atom, we mentioned that absorbing light with sufficient energy moves the electron to a higher energy level, and when it falls back to a lower energy level, light is emitted as energy is lost.
还记得在讨论氢原子的玻尔模型时,我们提到吸收具有足够能量的光会使电子跃迁到更高能级,而当电子回落到较低能级时,会发射光,因为能量被释放了。
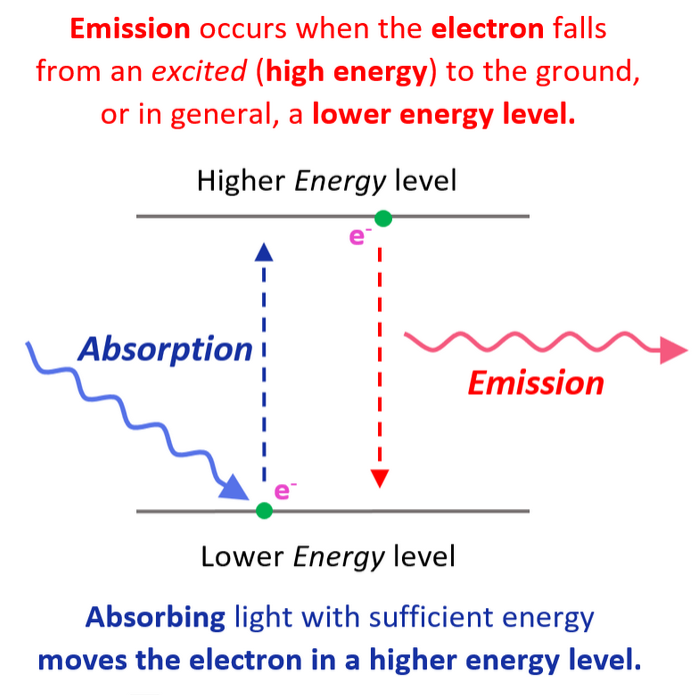
Now, when the electron is in a higher energy level than it normally is, the atom or ion is said to be in the excited state. If the electrons are in the lowest possible energy levels, the atom is in the ground state. So, everything we have discussed today pertains to the electron configurations of atoms at the ground state. In general, unless mentioned otherwise, the term electron configuration refers to the atom in the ground state.
现在,当电子处于比正常情况更高的能级时,原子或离子被称为处于激发态。如果电子处于可能的最低能级,原子则处于基态。因此,我们今天讨论的所有内容都与原子的基态电子构型有关。通常,除非另有说明,电子构型一词指的是原子的基态电子构型。
An example of switching from the ground to an excited state can be when the electron in a carbon atom jumps from the 2s to the 2p level. The electron configurations and orbital diagrams can be represented as:
从基态到激发态的一个例子是碳原子中的一个电子从 2s 轨道跃迁到 2p 轨道。其电子构型和轨道图可以表示为:

These electron transitions from lower to higher energy orbitals are the basis of many spectroscopies for determining the molecular structures in inorganic and organic materials.
这些电子从较低能级轨道到较高能级轨道的跃迁,是许多用于测定无机和有机材料分子结构的光谱学的基础。
Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle
泡利不相容原理、洪特规则和能量最低原理
Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle are rules for writing electron configurations, so go over the main principles of electron configurations if you need to before we start discussing them.
泡利不相容原理、洪特规则和能量最低原理是书写电子排布的规则,因此在我们开始讨论它们之前,如果需要的话,请先复习一下电子排布的主要原理。
Aufbau’s Principle
能量最低原理
Aufbau (German aufbauen, “to build up”) principle tells us that electrons fill the orbitals in the order of increasing their energy level. Remember, the main energy level is given by the principal quantum number, n, and it increases down the periodic table.
能量最低原理(德语“aufbauen”意为“构建”)告诉我们,电子按照能级升高的顺序填充轨道。请记住,主能级由主量子数 n 表示,并且在元素周期表中越往下,n 越大。

Here is a chart on the energy levels for up n = 4 which include the d sublevel:
以下是包含 d 亚层的、n 最大为 4 的能级图表:

Consider also that within the same principal level, orbitals with a lower value of l have lower energy (E) and therefore, are filled first. So, for a given value of n:
还要注意,在同一主能级中,角量子数 l 较小的轨道能量(E)较低,因此会先被填充。因此,对于给定的 n 值:
E (s orbital) < E (p orbital) < E (d orbital) < E (f orbital)
E(s 轨道)< E(p 轨道)< E(d 轨道)< E(f 轨道)
Notice that in the periodic table, it is opposite to what we show on the energy level diagram since the periods indicate the principal quantum number (n), which is the energy level, and they increase as we go down the periodic table. The general order for filling the electrons based on the energy levels and orbitals can be shown as in the picture below:
注意,在元素周期表中,情况与我们在能级图上所展示的相反,因为周期数表示主量子数(n),也就是能级,并且当我们沿着周期表向下移动时,周期数会增加。基于能级和轨道的电子填充一般顺序可以如下图所示:

Let’s look at a few examples of following the Aufbau principle. For example, hydrogen has one electron and of course, we are going to put it in the 1s orbital it has the lowest energy.
让我们看几个遵循能量最低原理的例子。例如,氢有一个电子,当然,我们会把它放在能量最低的1s 轨道中。

The electron configuration of hydrogen with orbital diagrams can be shown as:
氢的电子排布(含轨道图)可以表示为:

Next, we have helium, He – the second element in the periodic table. Remember, the number of electrons is equal to the atomic number as the number of electrons must be equal to the number of protons in a neutral atom. Therefore, He has two electrons and they both go to the 1s level because each orbital, regardless of if it is s, p, d, or f, can only accommodate a maximum of two electrons, and they wouldn’t go to the 2s level without filling the 1s:
接下来,我们看氦(He)——周期表中的第二个元素。请记住,在中性原子中,电子数等于原子序数,因为电子数必须等于质子数。因此,氦有两个电子,并且它们都会进入 1s 能级,因为每个轨道,无论它是 s、p、d 还是 f 轨道,最多只能容纳两个电子,而且在 1s 轨道未填满的情况下,电子不会进入 2s 能级:

The electron configuration of He with orbital diagrams can be shown as:
氦的电子排布(含轨道图)可以表示为:

And this keeps going by adding one more electron to the next lowest energy orbital for every subsequent element in the periodic table.
对于周期表中接下来的每个元素,都是通过向下一个能量最低的轨道再添加一个电子来进行电子填充的。
Pauli’s Exclusion Principle
泡利不相容原理
Notice that the arrow representing the second electron is pointing down, opposite to the first arrow which indicates that they must have opposite spins. This is according to the Pauli exclusion principle which states that no two electrons in an atom can have the same four quantum numbers. So, if the electrons are in the same orbital, they must have the same n, l, mₗ values, and therefore, the only one that can be different is the mₛ which is the spin of the electron shown by the position of the arrow.
注意,代表第二个电子的箭头指向下方,与第一个箭头方向相反,这表明它们必须具有相反的自旋。这是根据泡利不相容原理得出的,该原理指出,原子中没有两个电子可以具有相同的四个量子数。因此,如果电子处于同一轨道,它们的 n、l、mₗ 值必须相同,所以唯一可以不同的是 mₛ(即电子的自旋),这可以通过箭头的方向来表示。

Pauli’s Exclusion Principle
泡利不相容原理
No two electrons in an atom can have the same four quantum numbers.
一个原子中没有两个电子有相同的四个量子数。
This is because when they are in the same orbital, the values of n, /, and m are the same. Therefore, they must have opposite spins (different m,) so that they do not have all four quantum numbers the same.
这是因为当它们在同一轨道时,n, l 和 m 的值是相同的。因此,它们必须有相反的自旋(不同的m),这样它们的四个量子数就不一样了。
For example, for the electrons in a 2p orbital, n = 2, l = 1. and mₗ = –1, 0, or +1 (doesn’t matter which one because for the same orbital it will be identical). Therefore, the mₛ must be different (+1/2 or -1/2) so we don’t violate Pauli’s exclusion principle.
例如,对于 2p 轨道中的电子,n = 2,l = 1,mₗ = –1、0 或 +1(具体是哪个并不重要,因为对于同一轨道,mₗ 值是相同的)。因此,mₛ 必须不同(+1/2 或 -1/2),这样我们才不会违反泡利不相容原理。
The principle applies to all the orbitals. For example, notice how the electrons in each p orbital of oxygen, fluorine, and neon are with opposite spins:
该原理适用于所有轨道。例如,注意氧、氟和氖的每个 p 轨道中的电子都是相反自旋的:

Hund’s Rule
洪特规则
To understand Hund’s rule, let’s first write the electron configuration of boron:
为了理解洪特规则,让我们先写出硼的电子排布:

Now, the next electron, which indicates carbon, is going to have the option of pairing up with the one in the p orbital or going to the next empty p orbital. And it turns out that the electron goes to the next (empty) p orbital rather than fitting in with the other electron.
现在,下一个电子(代表碳元素)可以选择与 p 轨道中的那个电子配对,或者进入下一个空的 p 轨道。事实证明,电子会进入下一个(空的) p 轨道,而不是与另一个电子挤在一起。
This is the Hund’s rule, which states that electrons will fill all the degenerate orbitals (equal in energy) with parallel spins (both arrows up or down) first before pairing up in one orbital. We can also formulate it as the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons within the same energy sublevel.
这就是洪特规则,该规则指出,电子会先以平行自旋(箭头都向上或都向下) 填充所有简并轨道(能量相同的轨道),然后再在一个轨道中配对。我们也可以将其表述为:原子的最低能量排布是在同一能量亚层中具有最多未成对电子的排布。
Hund’s rule is another demonstration of the same principle which is the tendency to adopt the lowest energy state possible. There is a stronger repulsive interaction between two electrons in the same orbital compared to when they occupy separate orbitals of equal energy.
洪特规则是同一原理的另一种体现,即物质倾向于采取可能的最低能量状态。与两个电子占据能量相同的不同轨道相比,同一轨道中的两个电子之间存在更强的排斥作用。
Let’s show the application of Hund’s rule in explaining the electron configuration of carbon:
让我们展示洪特规则在解释碳的电子排布中的应用:

Hund’s Rule
洪特法则
The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons within the same energy sublevel.
原子的最低能量构型是在相同的能级内具有最多未配对电子的构型。
Do not place electrons in one orbital if there is an empty orbital of same energy.
如果有相同能量的空轨道,不要把电子放在一个轨道上。
Do not place it in the 3s orbital just for the sake of keeping a maximum number of unpaired electrons. It is energetically more favorable to have the electrons in the same lower sublevel rather than separating them by placing one in a higher energy level.
不要仅仅为了保留最大数量的未配对电子,而把它放在 3s 轨道上。从能量上讲,使电子处于同一较低的次能级比通过将一个电子置于较高能级来分离它们更有利。
Notice that placing the electron unpaired in the 3s orbital is also incorrect because, it is important to mention, that Hund’s rule applies to electrons in the same energy level. We are not going to place one of the electrons in the 3s orbital just for the sake of keeping a maximum number of unpaired electrons. It is energetically more favorable to have the electrons in the same lower sublevel rather than separating them by placing one in a higher energy level.
注意,将电子不成对地放在 3s 轨道中也是不正确的,因为需要强调的是,洪特规则适用于同一能级的电子。我们不会仅仅为了保持最多数量的未成对电子而将其中一个电子放在 3s 轨道中。从能量上讲,让电子处于同一个较低的亚层比将一个电子放在较高能级的轨道上而使它们分开更有利。
We can also demonstrate this using the orbital diagram of carbon:
我们也可以用碳的轨道图来证明这一点:

As expected, the next element, nitrogen will place the additional electron in the 3rd p orbital which is empty in carbon.
不出所料,下一个元素氮会将额外的电子放在碳中为空的第三个 p 轨道上。

Some exceptions occur in the electron configuration of d and f elements. First, recall that the (n +1)s orbitals always fill before the nd orbitals. For example, the 4s level fills before the 3d level, and therefore, the electron configuration of Ti is 1s²2s²2p⁶3s²3p⁶4s²3d² or, the condensed configuration which will be [Ar]4s²3d².
d 区和 f 区元素的电子排布存在一些例外情况。首先,请记住,(n + 1)s 轨道总是在 nd 轨道之前填充。例如,4s 能级先于 3d 能级填充,因此钛(Ti)的电子排布是 1s²2s²2p⁶3s²3p⁶4s²3d²,其简写排布为 [Ar]4s²3d²。

Electron Configuration of D Elements
D 元素的电子组态
The (n +1) s orbitals always fill before the nd orbitals as they are lower in energy.
(n +1) s 轨道总是在 nd 轨道之前填充,因为它们的能量较低。
Even though 3d has a smaller n value than 4s, we only start filling 3d once the 4s is complete.
即使 3d 具有比 4s 小的 n 值,我们只有在 4s 完成后才开始填充 3d。
Sometimes having half-filled orbitals compensate for the energy gain associated with placing the electron in a higher-level orbital.
有时,半满轨道的稳定性可以弥补将电子放在较高能级轨道所带来的能量损失。
For example, unlike the expected [Ar]4s²3d⁴, the electron configuration of Cr is [Ar]4s¹3d⁵, and the reason for this is that the d orbital gets a half-filled configuration (remember d orbitals can have a maximum of 10 electrons). We can think of this as the electron jumping from the 4s level to the 3d level and compensating this energy uphill by a stabilization associated with half-filled orbitals:
例如,与预期的 [Ar]4s²3d⁴ 不同,铬(Cr)的电子排布是 [Ar]4s¹3d⁵,原因是 d 轨道获得了半满构型(请记住,d 轨道最多可容纳 10 个电子)。我们可以认为,电子从 4s 能级跳到 3d 能级,而半满轨道的稳定性弥补了这一能量升高:

The Stability of Half-Filled Orbitals in Transition Metals
过渡金属中半填充轨道的稳定性
The electron jumps from the 4s level to the 3d level compensating the energy uphill by a stabilization associated with half-filled orbitals.
电子从 4s 能级跳到3d能级,通过与半填充轨道相关的稳定化来补偿向上的能量。
To summarize, remember that:
总结一下,请记住:
-
The Aufbau principle is about filling the orbitals from lower to higher energy
能量最低原理是指电子从低能量轨道向高能量轨道填充 -
Pauli’s exclusion principle tells us to place the arrows of electrons in the same orbital in opposite directions.
泡利不相容原理告诉我们,同一轨道中电子的箭头方向要相反。 -
Hund’s rule tells us to place the electrons in different orbitals of the same energy sublevel (s, p, d, f) rather than pairing them up – more unpaired electrons is better.
洪特规则告诉我们,电子要放在同一能量亚层(s、p、d、f)的不同轨道上,而不是配对——未成对电子越多越好。
Check this question, Multiple-Choice Quiz on the Electronic Structure of Atoms including questions on properties of light such as wavelength, frequency, energy, quantum numbers, atomic orbitals, electron configurations, and more.
查看下面的原子电子结构多项选择题测验,其中包括关于光的性质(如波长、频率、能量)、量子数、原子轨道、电子排布等问题。
Practice
练习题
1.
What is the maximum number of electrons that can occupy one p orbital?
一个 p 轨道最多能容纳多少个电子?
a. 14
b. 2
c. 10
d. 1
e. 6
答案:b
2.
The n = 2 shell can accommodate a maximum of ____ electrons.
n = 2 电子层最多能容纳 ____ 个电子。
a. 10
b. 8
c. 4
d. 6
e. 2
答案:b
3.
Which of the following is an incorrect orbital occupancy representation?
下列哪项是不正确的轨道占据表示?
a. 4d³
b. 3d⁵
c. 2s³
d. 1s¹
e. 2p²
答案:c
4.
The maximum number of electrons that can be accommodated in 3s subshell is
3s 亚层最多能容纳的电子数是
a. 10
b. 2
c. 6
d. 1
e. 8
答案:b
5.
The following electron configuration is incorrect:
下列电子排布不正确的是:
a. 1s²2s²
b. 1s²2s³2p³
c. 1s²2s²2p⁵
d. 1s²2s²2p⁴
e. 1s²2s²2p⁶3s²
答案:b
6.
Which electron configuration represents an excited state of the indicated atom?
下列哪项电子排布表示所指原子的激发态?
a. He: 1s²
b. Na: 1s² 2s² 2p⁶ 3s² 3p² 3s¹
c. P: 1s² 2s² 2p⁶ 3s² 3p³
d. N: 1s² 2s² 2p²3s¹
e. Ne: 1s² 2s² 2p⁶
答案:d
7.
This element is in the p-block of the periodic table
该元素位于周期表的 p 区
a. Cs
b. In
c. Pd
d. He
e. Po
答案:e
8.
Which of the following represents the ground state electron configuration of a transition element?
下列哪项表示过渡元素的基态电子排布?
a. 1s²2s²2p⁶3s²3p⁵
b. 1s² 2s² 2p⁶ 3s² 3p⁶3d¹⁰4s¹
c. 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁴
d. 1s²2s²2p⁶3s²3p⁶4s²
e. 1s²2s²2p³
答案:b
9.
Which of the following is the correct ground-state electron configuration of V is?
下列哪项是钒(V)正确的基态电子排布?
a. 1s²2s²2p⁶3s²3p⁶3d³
b. 1s²2s²2p⁶3s²3p⁶3d⁵
c. 1s²2s²2p⁶3s²4s²3d³
d. 1s²2s²2p⁶3s²3p⁶4s²3d⁵
e. 1s²2s²2p⁶3s²3p⁶4s²3d³
答案:e
10.
Which of the following represents a possible excited-state electron configuration for an iron atom?
下列哪项表示铁原子可能的激发态电子排布?
a. [Ar]3d⁷4s²
b. [Ar]3d⁶4s²
c. [Ar]3d⁷4s¹
d. [Ar]3d⁶4s¹
e. [Kr]3d⁷4s¹
答案:c
11.
Each of the following is an accurate representation of ground-state electron configuration except:
下列各项均是基态电子排布的正确表示,除了:
a. Fe: [Ar]4s²3d⁶.
b. Ca: [Ar]4s².
c. Se: [Ar] 4s²3d¹⁰4p⁴.
d. Ag: [Kr] 5s²4d⁹.
e. Ti: [Ar] 4s² 3d²
答案:d
12.
How many electrons, in total, are present in p orbitals in a ground-state nickel atom?
基态镍原子的 p 轨道中总共有多少个电子?
a. 8
b. 12
c. 6
d. 24
e. 3
答案:b
13.
Which one of the following represents electron configuration of an excited carbon atom?
下列哪项表示激发态碳原子的电子排布?
a. 1s²2s²2p³
b. 1s²2s²2p¹
c. 1s²2s²2p¹3s¹
d. 1s²2s²3s¹
e. 1s²2s²2p²
答案:c
14.
Which of the following ground-state electron configurations is incorrect?
下列哪项基态电子排布不正确?
a. Cl: [Ne]3s²3p⁵
b. Ge: [Ar] 4s² 3d¹⁰3p²
c. Co: [Ar]4s²3d⁷
d. Rb: [Kr]5s¹
e. Mn: [Ar] 4s²3d⁵
答案:b
15.
Bromine atom has ___ valence electrons.
溴原子有 ___ 个价电子。
a. 6
b. 4
c. 7
d. 5
e. 35
答案:c
16.
What is the ground-state electron configuration of chromium (Cr)?
铬(Cr)的基态电子排布是什么?
a. 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁶4d¹⁰5s²5p⁴
b. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵
c. 1s²2s²2p⁶3s²3p⁶3d¹⁰
d. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²3d⁴
e. 1s² 2s² 2p⁶ 3s² 3p⁶3d⁶
答案:b
17.
What is the ground-state electron configuration of bromine (Br)?
溴(Br)的基态电子排布是什么?
a. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰ 4p⁵
b. [Kr] 4s²3s¹⁰4p⁵
c. [Ne] 3s² 3p⁵
d. [Ar] 4s²3d¹⁰4p⁵
e. 1s² 2s² 2p⁵ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
答案:d
18.
The ground state electron configuration of As is ________.
砷(As)的基态电子排布是 ________。
a. 1s²2s²3s²3p⁶4s²3d¹⁰4p²
b. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p¹
c. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³
d. 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4d³
e. [Kr]4s²3d¹⁰4d³
答案:c
19.
Which of the following elements has the same valence-shell electron configuration as lithium?
下列哪种元素具有与锂相同的价层电子排布?
a. calcium.
b. sulfur.
c. potassium.
d. magnesium.
e. argon.
答案:c
20.
How many unpaired electrons are found in the d orbital of nickel.
镍的 d 轨道中有多少个未成对电子。
a. 4 electrons
b. 3 electrons
c. 2 electrons
d. 8 electrons
e. none of these
答案:c
21.
The ground-state electron configuration of the element ________ is [Kr]5s²4d¹⁰
元素 ________ 的基态电子排布是 [Kr]5s²4d¹⁰
a. Fe
b. Cd
c. Cr
d. Mn
e. Zn
答案:b
22.
The ground-state electron configuration of ________ is [Ar]4s²3d⁷.
________ 的基态电子排布是 [Ar]4s²3d⁷。
a. Ti
b. Co
c. Fe
d. Cr
e. Cs
答案:b
23.
Chlorine has the following ground-state configuration:
氯具有以下基态排布:
a. [He]3s²3p²
b. [Ne]3s²3p⁵
c. [He]2s²2p⁵
d. [Ne]2s²2p³
e. [He]3s²3p⁵
答案:b
24.
What is the maximum number of electrons that can be accommodated in each d-subshell?
每个 d 亚层最多能容纳多少个电子?
a. 10
b. 5
c. 6
d. 3
e. 2
答案:a
25.
The 3p subshell of a sulfur atom contains ________ electrons.
硫原子的 3p 亚层含有 ________ 个电子。
a. 16
b. 8
c. 2
d. 4
e. 6
答案:d
26.
What noble gas core should be used when writing the condensed electron configuration of Strontium (Sr)?
书写锶(Sr)的简写电子排布时,应使用哪种稀有气体核?
a. [Xe]
b. [Kr]
c. [Ne]
d. [Ar]
e. [He]
答案:b
27.
Electron Configuration of Ions
离子的电子排布
Which of the following is the ground state electron configuration of S²⁻?
下列哪项是 S²⁻ 的基态电子排布?
a) [Ne]
b) [Ne]3s²3p⁶
c) [Ne]3s²3p⁴
d) [Ne]3s²3p²
e) [Ne]4s²
答案:b
28.
A cation of +3 indicates that an element has
+3 价阳离子表明一种元素
a) lost three neutrons.
b) lost three protons.
c) lost three electrons.
d) gained three protons.
e) gained three electrons.
答案:c
29.
Indicate the ground state electron configuration for Cl⁻.
指出 Cl⁻ 的基态电子排布。
a) 1s²2s²2p⁶3s²3p⁴
b) 1s²2s²2p⁶3s²3p⁵
c) 1s²2s²2p⁶3s²3p⁶
d) 1s²2s²2p⁶3s¹3p⁶
e) 1s²2s²2p⁶3s³3p⁵
答案:c
30.
Which of the following is the ground state electron configuration of Ba²⁺ ?
下列哪项是 Ba²⁺ 的基态电子排布?
a) [Kr]5s²4d¹⁰5p⁶6s²
b) [Kr]5s²4d¹⁰5p⁶
c) [Kr]5s²4d¹⁰5p⁶5d²
d) [Kr]5s²5p⁶
e) [Kr]5s²4d¹⁰5p⁶6s²
答案:b
31.
Which of the following is the ground state electron configuration of Ti²⁺.
下列哪项是 Ti²⁺ 的基态电子排布。
a) [Ar]3d⁴
b) [Ar]3d²
c) [Ar]4s²
d) [Ar]4s²3d⁴
e) [Ar]4s²3d²
答案:b
32.
Which of the following is the ground state electron configuration of Zn²⁺?
下列哪项是 Zn²⁺ 的基态电子排布?
a) [Ar]4s²3d⁶
b) [Ar]3d¹⁰
c) [Ar]4s²3d⁸
d) [Ar]3d⁸
e) [Ar]
答案:b
33.
Which of the following is the ground state electron configuration of Cr³⁺?
下列哪项是 Cr³⁺ 的基态电子排布?
a) [Ar]
b) [Ar]4s¹3d²
c) [Ar]3d³
d) [Ar]4s²3d¹
e) [Ar]4s²3d⁷
答案:c
34.
How many valence electrons are present in the azide (N₃⁻) ion?
叠氮离子(N₃⁻)中有多少个价电子?
a) 3
b) 5
c) 8
d) 7
e) 2
答案:c
35.
Ca²⁺ has the following ground state electron configuration:
Ca²⁺ 具有以下基态电子排布:
a) 1s²2s²2p⁶
b) 1s²2s²2p⁶3s²
c) 1s²2s²2p⁶3s²3p²
d) 1s²2s²2p⁶3s²3p⁶4s²3d²
e) 1s²2s²2p⁶3s²3p⁶
答案:e
36.
Rb⁺ has the following ground state electron configuration:
Rb⁺ 具有以下基态电子排布:
a) [Kr]5s²4d⁶
b) [Kr]4s²
c) [Ar]4s²4p⁴
d) [Kr]5s¹
e) [Ar]4s²3d¹⁰4p⁶
答案:e
37.
Which of the following is the ground state electron configuration of Fe³⁺?
下列哪项是 Fe³⁺ 的基态电子排布?
a) [Ar]3d⁵
b) [Ar]4s¹3d³
c) [Ar]
d) [Ar]4s²3d⁹
e) [Ar]4s²3d¹
答案:a
38.
The correct ground state electron configuration for Br⁻ is:
Br⁻ 正确的基态电子排布是:
a) [Ar]4s²3d¹⁰4p⁶
b) [Ar]4s²3d¹⁰4p³
c) [Ar]4s²3d⁸4p⁶
d) [Ar]4s²3d¹⁰4p⁵
e) [Ar]4s²4p⁶
答案:a
39.
How many electrons are present in the ground state of Mg²⁺ ion?
基态 Mg²⁺ 离子中有多少个电子?
a) 2
b) 6
c) 10
d) 8
e) 12
答案:c
via:
- Hund’s Rule Definition and Examples
https://sciencenotes.org/hunds-rule-definition-and-examples/ - Electron Configurations - Chemistry Steps
https://general.chemistrysteps.com/electron-configurations/ - Hund’s Rule - Chemistry Steps
https://general.chemistrysteps.com/hunds-rule/ - Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle - Chemistry Steps
https://general.chemistrysteps.com/aufbau-principle-hunds-rule-paulis-principle/





















 6万+
6万+

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








