注:本文为 “原子轨道” 相关合辑。
英文引文,机翻未校。
中文引文,略作重排。
如有内容异常,请看原文。
Why atomic orbits are named as k, l, m and n and so on?
为什么原子轨道被命名为 k、l、m、n 等等?
Nandakishore Ravi
Jul 3, 2023
In your chemistry class when you are learning the atomic model, if you are attentive enough you might have noticed that the various orbits where electrons revolve around the central nucleus are labelled as k, l, m and so on. Why can’t they be named a, b, c and so on?
在化学课上学习原子模型时,如果你足够用心,可能会注意到电子绕中心原子核旋转的各种轨道被标记为 k、l、m 等等。为什么不能用 a、b、c 等等来命名呢?
So, the story goes like this…
事情是这样的……
Charles Barkla’s Discovery
查尔斯·巴克拉的发现
Once there lived a spectroscopist named Charles Barkla, who studied characteristic x-rays emitted when an atom is bombarded with high energy electrons.
曾经有一位名叫查尔斯·巴克拉(Charles Barkla)的光谱学家,他研究原子被高能电子轰击时发出的特征X射线。
Wait a second… At this point you might have got another question in your mind.
等一下……看到这里,你脑子里可能会冒出另一个问题。
Why X-ray is named as it is
为什么 X 射线被命名为 X 射线
“Why was X ray named as it is and not Y or Z rays?”
“为什么 X 射线被命名为 X 射线,而不是Y射线或Z射线呢?”

Photo by CDC on Unsplash
Well, the term X ray was initially called X radiation, coined by German Physicist Wilhelm Roentgen. “X” in the name actually denotes an unknown type, as one shall use
x
x
x as a variable for defining an unknown quantity.
其实,X 射线一词最初被称为 X 辐射,由德国物理学家威廉·伦琴(Wilhelm Roentgen)创造。名称中的 “X” 实际上表示一种未知类型,就像人们用
x
x
x 作为变量来定义未知量一样。
Back to Barkla’s Discovery
回到巴克拉的发现
While studying X-rays emitted from excited atoms, he saw mostly two types of rays. One with high energy, he called as “A” and the other with lower energy, he called “B”. He later thought his “A” might not be the highest energy ray and in order to provide room for future discoveries, he later renamed it as “K” and the other as “L”.
在研究受激原子发出的 X 射线时,他主要观察到两种射线。一种能量较高,他称之为 “A”;另一种能量较低,他称之为 “B”。后来,他认为自己发现的 “A” 可能不是能量最高的射线,而且为了给未来的发现留出空间,他后来将其重新命名为 “K”,另一种则命名为“L”。
It was later found that the high energy K-rays were emitted when electrons were released free from the innermost orbit upon interaction with bombarded electrons were later recaptured.
后来发现,当电子与轰击电子相互作用而从最内层轨道释放出来,之后又被重新捕获时,就会发出高能的K射线。
This scientific convention has also led Bohr to name his atomic orbits as k, l, m and n respectively.
这一科学惯例也促使玻尔(Bohr)将他提出的原子轨道分别命名为 k、l、m 和 n。

Photo by Norbert Kowalczyk on Unsplash
Hope this might help you in naming your discoveries too in future.
希望这也能帮助你在未来为自己的发现命名。
Author Information
作者信息
Written by Nandakishore Ravi
作者:南达基肖尔·拉维(Nandakishore Ravi)
✨️ PhD student (ECE), Development of Sustainable energy technology 🔋🫧🔬
Electron Shells: Understanding the Atom’s Architecture
电子能阶:了解原子的结构
By Sania Anwar
Date: January 16, 2025
The atom, the fundamental building block of all matter, is a complex and fascinating entity. While it’s often depicted as a simple sphere, its internal structure is far more intricate. At the heart of the atom lies the nucleus, composed of protons and neutrons. Surrounding this nucleus is a cloud of negatively charged particles known as electrons. These electrons don’t orbit the nucleus like planets around a star, but rather exist in regions of space called electron [shells](https://in.pinterest.com/search/pins/?q=Fern future home vibes).
原子是所有物质的基本构成单位,是一个复杂而迷人的实体。虽然它常被描绘成一个简单的球体,但其内部结构要复杂得多。原子的核心是原子核,由质子和中子组成。围绕原子核的是一团带负电的粒子,称为电子。这些电子并非像行星绕恒星一样绕原子核运行,而是存在于被称为电子能层的空间区域中。
Understanding Electron Shells
了解电子能层
Imagine an onion. It has layers, right? Similarly, electron shells are like concentric layers surrounding the nucleus. Each shell represents a specific energy level for the electrons.
想象一下洋葱,它有多层结构,对吧?同样,电子能层就像围绕原子核的同心层。每个能层代表电子的一个特定能量级。
Shell 1 (K-shell): This is the innermost shell and can hold a maximum of 2 electrons.
1 能层(K 能层): 这是最内层的能层,最多可容纳 2 个电子。
Shell 2 (L-shell): The second shell can accommodate up to 8 electrons.
2 能层(L 能层): 第二层能层最多可容纳 8 个电子。
Shell 3 (M-shell): This shell can hold a maximum of 18 electrons.
3 能层(M 能层): 这个能层最多可容纳 18 个电子。
Shell 4 (N-shell): The fourth shell can accommodate up to 32 electrons.
4 能层(N 能层): 第四层能层最多可容纳 32 个电子。
And so on. The number of electrons that can reside in each shell is determined by a complex set of rules governed by quantum mechanics.
以此类推。每个能层可容纳的电子数量由量子力学所支配的一系列复杂规则决定。
Electron Configuration and the Periodic Table
电子排布与元素周期表
The arrangement of electrons within these shells is crucial for understanding the properties of elements. This arrangement is known as electron configuration.
这些能层中电子的排布对于理解元素的性质至关重要。这种排布被称为电子构型。
The Aufbau Principle
泡利不相容原理
This principle states that electrons fill the lowest energy levels first. So, the first two electrons go into the K-shell, the next eight into the L-shell, and so on.
这一原理指出,电子优先填充能量最低的能级。因此,前两个电子进入 K 能层,接下来的八个电子进入 L 能层,依此类推。
Hund’s Rule
洪德规则
When electrons occupy orbitals within a subshell, they tend to fill each orbital singly with parallel spins before pairing up.
当电子占据亚能层内的轨道时,它们倾向于先以平行自旋单独填充每个轨道,然后再配对。
Pauli Exclusion Principle
泡利不相容原理
No two electrons in an atom can have the same set of four quantum numbers.
一个原子中没有两个电子可以具有相同的一组四个量子数。
The periodic table, a cornerstone of chemistry, reflects the electron configurations of elements. Elements in the same group (vertical column) have similar electron configurations in their outermost shell, which explains their shared chemical properties.
元素周期表是化学的基石,它反映了元素的电子构型。同一族(垂直列)中的元素在最外层能层具有相似的电子构型,这解释了它们具有相似的化学性质。
The Importance of Electron Shells
电子能层的重要性
Chemical Bonding
化学键合
The arrangement of electrons in the outermost shell, also known as the valence shell, determines how an atom interacts with other atoms. Atoms tend to gain, lose, or share electrons to achieve a stable configuration, usually with a full outer shell. This drives the formation of chemical bonds, leading to the creation of molecules and compounds.
最外层能层(也称为价能层)中电子的排布决定了一个原子与其他原子的相互作用方式。原子倾向于获得、失去或共享电子以达到稳定构型,通常是使最外层能层充满电子。这推动了化学键的形成,进而形成分子和化合物。
Atomic Size
原子大小
The number of electron shells in an atom significantly influences its size. As you move down a group in the periodic table, the number of shells increases, leading to larger atomic radii.
原子中电子能层的数量对其大小有显著影响。在元素周期表中,当你沿着一族向下移动时,能层数量增加,导致原子半径增大。
Ionization Energy
电离能
The energy required to remove an electron from an atom is called ionization energy. Electrons in outer shells have lower ionization energies than those closer to the nucleus.
从原子中移除一个电子所需的能量称为电离能。外层能层中的电子比靠近原子核的电子具有更低的电离能。
Electronegativity
电负性
This property measures an atom’s tendency to attract electrons towards itself in a chemical bond. Electronegativity generally increases across a period and decreases down a group, which is related to the effective nuclear charge experienced by the valence electrons.
这一性质衡量原子在化学键中吸引电子向自身的倾向。电负性通常在同一周期中从左到右递增,在同一族中从上到下递减,这与价电子所感受到的有效核电荷有关。
FAQs
常见问题
What is an electron shell?
什么是电子能层?
An electron shell is a grouping of electrons surrounding an atom’s nucleus, characterized by a principal quantum number (
n
n
n). Each shell represents an energy level where electrons reside.
电子能层是围绕原子核的一组电子,由主量子数(
n
n
n)表征。每个能层代表电子所处的一个能量级。
How are electron shells labeled?
电子能层如何标记?
Electron shells are labeled numerically (1, 2, 3, etc.) or alphabetically using letters K, L, M, N, and so on, corresponding to
n
=
1
,
2
,
3
,
4
n = 1, 2, 3, 4
n=1,2,3,4, respectively.
电子能层可以用数字(1、2、3 等)标记,也可以用字母 K、L、M、N 等按字母顺序标记,分别对应
n
=
1
、
2
、
3
、
4
n = 1、2、3、4
n=1、2、3、4。
How many electrons can each shell hold?
每个能层可以容纳多少个电子?
The maximum number of electrons in a shell is determined by the formula
2
n
2
2n²
2n2,where
n
n
n is the principal quantum number:
一个能层中最多可容纳的电子数由公式
2
n
2
2n²
2n2 确定,其中
n
n
n 是主量子数:
K shell (
n
=
1
n=1
n=1): 2 electrons
K 能层(
n
=
1
n=1
n=1): 2 个电子
L shell (
n
=
2
n=2
n=2): 8 electrons
L 能层(
n
=
2
n=2
n=2): 8 个电子
M shell (
n
=
3
n=3
n=3): 18 electrons
M 能层(
n
=
3
n=3
n=3): 18 个电子
N shell (
n
=
4
n=4
n=4): 32 electrons
N 能层(
n
=
4
n=4
n=4): 32 个电子
What are subshells and orbitals?
什么是亚能层和轨道?
Each electron shell comprises subshells (s, p, d, f), which are further divided into atomic orbitals. Subshells are defined by the azimuthal quantum number (
ℓ
\ell
ℓ),and each type can hold a specific maximum number of electrons:
每个电子能层包含亚能层(s、p、d、f),亚能层又进一步分为原子轨道。亚能层由角量子数(
ℓ
\ell
ℓ)定义,每种类型的亚能层可容纳特定数量的最大电子数:
s subshell (
ℓ
=
0
\ell=0
ℓ=0): 2 electrons
s 亚能层(
ℓ
=
0
\ell=0
ℓ=0): 2 个电子
p subshell (
ℓ
=
1
\ell=1
ℓ=1): 6 electrons
p 亚能层(
ℓ
=
1
\ell=1
ℓ=1): 6 个电子
d subshell (
ℓ
=
2
\ell=2
ℓ=2): 10 electrons
d 亚能层(
ℓ
=
2
\ell=2
ℓ=2): 10 个电子
f subshell (
ℓ
=
3
\ell=3
ℓ=3): 14 electrons
f 亚能层(
ℓ
=
3
\ell=3
ℓ=3): 14 个电子
How do electrons fill these shells and subshells?
电子如何填充这些能层和亚能层?
Electrons occupy the lowest available energy levels first, following the Aufbau principle. The sequence is guided by the
n
+
ℓ
n + \ell
n+ℓ rule, where subshells with lower
n
+
ℓ
n + \ell
n+ℓ values are filled before those with higher values. In cases of equal
n
+
ℓ
n + \ell
n+ℓ values, the subshell with a lower
n
n
n value is filled first.
遵循泡利原理,电子首先占据能量最低的可用能级。填充顺序由
n
+
ℓ
n + \ell
n+ℓ 规则指导,即
n
+
ℓ
n + \ell
n+ℓ 值较小的亚能层先于
n
+
ℓ
n + \ell
n+ℓ 值较大的亚能层被填充。当
n
+
ℓ
n + \ell
n+ℓ 值相等时,
n
n
n 值较小的亚能层先被填充。
What is the significance of valence electrons?
价电子的重要性是什么?
Valence electrons are the electrons in the outermost shell of an atom. They play a crucial role in chemical bonding and reactions, as atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration.
价电子是原子最外层能层中的电子。它们在化学键合和化学反应中起着至关重要的作用,因为原子倾向于获得、失去或共享价电子以达到稳定的电子构型。
Why are electron shells named K, L, M, N instead of A, B, C?
为什么电子能层命名为 K、L、M、N 而不是 A、B、C?
The naming convention (K, L, M, N) originates from early X-ray spectroscopy studies. The initial labels started with K to avoid confusion with other spectral lines labeled A, B, C in different contexts.
这种命名惯例(K、L、M、N)源于早期的 X 射线光谱学研究。最初从 K 开始标记是为了避免与不同情况下标记为 A、B、C 的其他谱线混淆。
How do electron shells relate to the periodic table?
电子能层与元素周期表有什么关系?
Each row (period) in the periodic table corresponds to the filling of a new electron shell. Elements in the same column (group) have similar valence electron configurations, leading to comparable chemical properties.
元素周期表中的每一行(周期)对应于一个新电子能层的填充。同一列(族)中的元素具有相似的价电子构型,因此具有相似的化学性质。
Can electron shells overlap in energy?
电子能层在能量上会重叠吗?
Yes, especially in larger atoms. For instance, the 4s subshell has a lower energy than the 3d subshell, so it fills first. This overlap is due to the specific solutions to the Schrödinger equation for multi-electron atoms.
是的,特别是在较大的原子中。例如,4s 亚能层的能量低于 3d 亚能层,因此它先被填充。这种重叠是由于多电子原子的薛定谔方程的特定解所致。
How do electron shells influence chemical bonding?
电子能层如何影响化学键合?
The arrangement of electrons in shells determines how atoms interact. Atoms with incomplete valence shells tend to form bonds to achieve stability, leading to the formation of molecules and compounds.
能层中电子的排布决定了原子之间的相互作用方式。价能层未填满的原子倾向于形成键以达到稳定状态,从而形成分子和化合物。
To conclude
总结
Understanding electron shells is fundamental to grasping atomic structure and chemical behavior. Electron shells, defined by principal quantum numbers, organize electrons into energy levels around an atom’s nucleus. Each shell can hold a specific maximum number of electrons, determined by the formula
2
n
2
2n²
2n2。These shells are further divided into subshells and orbitals, each with distinct shapes and capacities.
理解电子能层是掌握原子结构和化学行为的基础。由主量子数定义的电子能层将电子组织在原子核周围的不同能量级上。每个能层可容纳的最大电子数由公式
2
n
2
2n²
2n2 确定。这些能层进一步分为亚能层和轨道,每个都有独特的形状和容量。
The arrangement of electrons within these shells and subshells dictates how atoms interact, bond, and react with one another. Valence electrons, located in the outermost shell, are particularly significant as they determine an element’s chemical properties and its tendency to engage in chemical reactions.
这些能层和亚能层中电子的排布决定了原子如何相互作用、成键和反应。位于最外层能层的价电子尤为重要,因为它们决定了元素的化学性质及其参与化学反应的倾向。
The periodic table is structured in a way that reflects the filling of electron shells. Each period corresponds to the addition of a new electron shell, and elements within the same group share similar valence electron configurations, leading to comparable chemical behaviors.
元素周期表的结构反映了电子能层的填充情况。每个周期对应于一个新电子能层的增加,同一族中的元素具有相似的价电子构型,因此具有相似的化学行为。
It’s also noteworthy that electron shells are labeled using letters (K, L, M, N) rather than starting with A. This convention stems from early spectroscopic studies and has been retained to avoid confusion with other labeling systems.
值得注意的是,电子能层使用字母(K、L、M、N)标记,而不是从 A 开始。这一惯例源于早期的光谱学研究,并被保留下来以避免与其他标记系统混淆。
In summary, electron shells provide a framework for understanding the distribution of electrons in an atom, which in turn explains the atom’s chemical properties and its interactions with other atoms. A solid grasp of this concept is essential for delving deeper into the fields of chemistry and atomic physics.
总之,电子能层为理解原子中电子的分布提供了一个框架,进而解释了原子的化学性质及其与其他原子的相互作用。牢固掌握这一概念对于深入研究化学和原子物理学领域至关重要。
What are Shells?
什么是能层?
According to Bohr’s Atomic model electrons revolve around the nucleus in a specific circular path known as orbit or called a shell. Shells have stationary energy levels, the energy of each shell is constant.
根据玻尔原子模型,电子在特定的圆形路径上绕原子核旋转,这种路径被称为轨道或能层。能层具有固定的能级,每个能层的能量是恒定的。
Each stationary orbit or shell is associated with a definite amount of energy. The greater the distance of the orbit from the nucleus, the more shall be the energy associated with it. These shells are called energy levels. It is numbered as 1, 2, 3, 4, ……. or K, L, M, N, …… from the nucleus outwards.
每个固定的轨道或能层都与一定的能量相关联。轨道离原子核越远,与之相关的能量就越大。这些能层被称为能级。从原子核向外,它们被编号为 1、2、3、4……或 K、L、M、N……
An electron shell may contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell.
一个电子能层只能包含固定数量的电子,每个能层都与特定范围的电子能量相关联,因此必须先填满一个能层,才能将电子添加到外层能层。
Characteristic of Shells
能层的特征
Principal Quantum Number
主量子数
- The principal quantum number is denoted by “ n n n”。It represents the name, size and energy of the shell to which the electron belongs. The value of n n n lies between 1 to ∞ \infty ∞。
- 主量子数 用“ n n n”表示。它代表电子所属能层的名称、大小和能量。 n n n 的值在 1 到 ∞ \infty ∞ 之间。

| The value of “ n n n” | Designation of shell |
|---|---|
| 1 | K |
| 2 | L |
| 3 | M |
| 4 | N |
| 5 | O |
| 6 | P |
| 7 | Q |
Distance and Energy of Shells
能层的距离和能量
-
Higher the value of “ n n n” the greater the distance of the shell from the nucleus.
“ n n n”的值越大,能层离原子核的距离越远。
r 1 < r 2 < r 3 < r 4 < ⋯ r_1 < r_2 < r_3 < r_4 < \cdots r1<r2<r3<r4<⋯r = ( n 2 Z ) × 0.529 A ˚ r = \left( \dfrac{n^2}{Z} \right) \times 0.529 \ \text{Å} r=(Zn2)×0.529 A˚
-
Greater the value of “ n n n” higher the magnitude of energy.
“ n n n”的值越大,能量的量级越高。
E 1 < E 2 < E 3 < E 4 < E 5 < ⋯ E_1 < E_2 < E_3 < E_4 < E_5 < \cdots E1<E2<E3<E4<E5<⋯ -
Energy separation between two shells decreases on moving away from the nucleus.
随着离原子核距离的增加,两个能层之间的能量差减小。
( E 2 − E 1 ) > ( E 3 − E 2 ) > ( E 4 − E 3 ) > ⋯ (E_2 - E_1) > (E_3 - E_2) > (E_4 - E_3) > \cdots (E2−E1)>(E3−E2)>(E4−E3)>⋯
Maximum Number of Electrons in a Shell
每个能层最多可容纳的电子数
-
The maximum number of electrons present in the shell on the 2 n 2 2n^2 2n2 rule.
能层中最多能容纳的电子数遵循 2 n 2 2n^2 2n2 规则。Name of Shell The value of “ n n n” Maximum electrons present 2 n 2 2n^2 2n2 K 1 2 L 2 8 M 3 18 N 4 32 -
The angular momentum of each shell can be calculated by the formula.
每个能层的角动量可以通过以下公式计算:
m v r = n h 2 π mvr = \dfrac{nh}{2\pi} mvr=2πnh
Definition of Subshell
亚能层的定义
All the electrons having the same shell do not have the same energy. Based on the energy of the electrons, the shells are divided into sublevels or subshells. Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals.
同一能层中的所有电子能量并不相同。根据电子的能量,能层被分为不同的能级或亚能层。每个能层由一个或多个亚能层组成,而亚能层本身又由原子轨道组成。
Azimuthal Quantum Number
角量子数
-
The subshells are described with the help of Azimuthal quantum number ℓ \ell ℓ。
亚能层借助角量子数( ℓ \ell ℓ)来描述。 -
The value of “ ℓ \ell ℓ” depends upon the value of the shell ( n n n) with which it is associated.
“ ℓ \ell ℓ” 的值取决于与其相关联的能层( n n n)的值。 -
These subshells have been designated as s ( ℓ = 0 \ell = 0 ℓ=0),p ( ℓ = 1 \ell =1 ℓ=1),d ( ℓ = 2 \ell = 2 ℓ=2) and f ( ℓ = 3 \ell =3 ℓ=3)。
这些亚能层被命名为s( ℓ = 0 \ell = 0 ℓ=0)、p( ℓ = 1 \ell = 1 ℓ=1)、d( ℓ = 2 \ell = 2 ℓ=2)和f( ℓ = 3 \ell = 3 ℓ=3)。
Energy Order of Subshells
亚能层的能量顺序
- The energies of the various subshells in the same shell are in the order
s
<
p
<
d
<
f
s < p < d < f
s<p<d<f。
同一能层中不同亚能层的能量顺序为 s < p < d < f s < p < d < f s<p<d<f。
Size of Subshells
亚能层的尺寸
- The subshell having equal “
ℓ
\ell
ℓ” value but with different
n
n
n values have similar shape but their size increases as the value of
n
n
n increases.
2
s
2s
2s-subshell is greater in size than
1
s
1s
1s-subshell.
ℓ \ell ℓ 值相同但 n n n 值不同的亚能层具有相似的形状,但随着 n n n 值的增大,其尺寸也会增大。 2 s 2s 2s 亚能层的尺寸大于 1 s 1s 1s 亚能层。
Maximum Number of Electrons in a Subshell
每个亚能层最多能容纳的电子数
-
The maximum electrons present in a subshell = 2 ( 2 ℓ + 1 ) 2(2\ell +1) 2(2ℓ+1)。
一个亚能层中最多能容纳的电子数 = 2 ( 2 ℓ + 1 ) 2(2\ell + 1) 2(2ℓ+1)。Subshell Maximum electrons The historical name of subshell s 2 sharp p 6 principal d 10 diffuse f 14 fundamental
Number of Electrons in Each Shell
每个能层中的电子数量
-
Each shell contains one or more subshells.
每个能层包含一个或多个亚能层。 -
K shell contains only one subshell – 1s
K 能层只包含一个亚能层——1s -
L shell contains two subshells – 2s, 2p
L 能层包含两个亚能层——2s、2p -
M shell contains three subshells – 3s, 3p, 3d
M 能层包含三个亚能层——3s、3p、3d -
N shell contains four subshells – 4s, 4p, 4d, 4f
N 能层包含四个亚能层——4s、4p、4d、4f
Electron Distribution in Shells and Subshells
电子在能层和亚能层中的排布
The electrons are arranged in the shell in the following manner:
电子在能层中的排布方式如下:
| Name of the shell | Name of the subshell | Maximum no. of electrons present in the shell | Distribute the electron in the subshell |
|---|---|---|---|
| K | 1s | 2 | 1s² |
| L | 2s, 2p | 8 | 2s² 2p⁶ |
| M | 3s, 3p, 3d | 18 | 3s², 3p⁶, 3d¹⁰ |
| N | 4s, 4p, 4d, 4f | 32 | 4s², 4p⁶, 4d¹⁰, 4f¹⁴ |
The Order of Energy of the Subshell
亚能层的能量顺序
The filling of the shells and subshells with electrons proceeds from subshells of lower energy to subshells of higher energy. This follows the Aufbau principle.
电子在能层和亚能层中的填充是从能量较低的亚能层到能量较高的亚能层进行的。这遵循泡利原理。
n + ℓ n + \ell n+ℓ Rule
n + ℓ n + \ell n+ℓ 规则
According to this subshells with a lower
n
+
ℓ
n + \ell
n+ℓ value are filled before those with higher
n
+
ℓ
n + \ell
n+ℓ values. In the case of equal
n
+
ℓ
n + \ell
n+ℓ values, the subshell with a lower
n
n
n value is filled first.
根据这一原理,
n
+
ℓ
n + \ell
n+ℓ 值较低的亚能层比
n
+
ℓ
n + \ell
n+ℓ 值较高的亚能层先被填充。当
n
+
ℓ
n + \ell
n+ℓ 值相等时,
n
n
n 值较低的亚能层先被填充。
A subshell that has a lower
n
+
ℓ
n + \ell
n+ℓ means lower energy. Among 4s and 3d;
n
+
ℓ
n + \ell
n+ℓ 值较低的亚能层意味着能量较低。在 4s 和 3d 中:
4 s ; ( n + ℓ ) = ( 4 + 0 ) = 4 4s ; (n + \ell) = (4 + 0) = 4 4s;(n+ℓ)=(4+0)=4
3 d ; ( n + ℓ ) = ( 3 + 2 ) = 5 3d ; (n + \ell) = (3 + 2) = 5 3d;(n+ℓ)=(3+2)=5
4s < 3d (energy)
The energy order of the subshells is given below
亚能层的能量顺序如下:

Arrangement of Electrons in the Shell and Subshell
电子在能层和亚能层中的排布
The first 20 element electrons are arranged in shell and subshell according to their energy level in the below table.
下表是前 20 种元素的电子根据其能级在能层和亚能层中的排布情况。
| Atomic Number | Element Name | K | L | M | N |
|---|---|---|---|---|---|
| 1 | Hydrogen (H) | 1s¹ | |||
| 2 | Helium (He) | 1s² | |||
| 3 | Lithium (Li) | 1s² | 2s¹ | ||
| 4 | Beryllium (Be) | 1s² | 2s² | ||
| 5 | Boron (B) | 1s² | 2s² | 2p¹ | |
| 6 | Carbon © | 1s² | 2s² | 2p² | |
| 7 | Nitrogen (N) | 1s² | 2s² | 2p³ | |
| 8 | Oxygen (O) | 1s² | 2s² | 2p⁴ | |
| 9 | Fluorine (F) | 1s² | 2s² | 2p⁵ | |
| 10 | Neon (Ne) | 1s² | 2s² | 2p⁶ | |
| 11 | Sodium (Na) | 1s² | 2s² | 2p⁶ | 3s¹ |
| 12 | Magnesium (Mg) | 1s² | 2s² | 2p⁶ | 3s² |
| 13 | Aluminium (Al) | 1s² | 2s² | 2p⁶ | 3s² 3p¹ |
| 14 | Silicon (Si) | 1s² | 2s² | 2p⁶ | 3s² 3p² |
| 15 | Phosphorous § | 1s² | 2s² | 2p⁶ | 3s² 3p³ |
| 16 | Sulfur (S) | 1s² | 2s² | 2p⁶ | 3s² 3p⁴ |
| 17 | Chlorine (Cl) | 1s² | 2s² | 2p⁶ | 3s² 3p⁵ |
| 18 | Argon (Ar) | 1s² | 2s² | 2p⁶ | 3s² 3p⁶ |
| 19 | Potassium (K) | 1s² | 2s² | 2p⁶ | 3s² 3p⁶ 4s¹ |
| 20 | Calcium (Ca) | 1s² | 2s² | 2p⁶ | 3s² 3p⁶ 4s² |
Frequently Asked Questions on Shells
关于能层的常见问题
Q1: What are energy shells in chemistry?
化学中的能层是什么?
The energy shell is associated with a definite amount of energy. The greater the distance of the orbit from the nucleus, the more shall be the energy associated with it. These shells are called energy levels.
能层与一定的能量相关联。轨道离原子核越远,与之相关的能量就越多。这些能层被称为能级。
Q2: What are KLM and N shells in chemistry?
化学中的 K、L、M 和 N 能层是什么?
The K shell is the first shell or first energy level, L is the second shell and 2nd energy level, and M is the third shell and 3rd energy level. N is the fourth shell and 4th energy level.
K 能层是第一个能层或第一能级,L 是第二个能层或第二能级,M 是第三个能层或第三能级,N 是第四个能层或第四能级。
Q3: How do electrons fill the shell?
电子如何填充能层?
The filling of the electrons in the shells proceeds from the lower energy to higher energy. According to this subshells with a lower
n
+
ℓ
n + \ell
n+ℓ value are filled before those with higher
n
+
ℓ
n + \ell
n+ℓ values. In the case of equal
n
+
ℓ
n + \ell
n+ℓ values, the subshell with a lower
n
n
n value is filled first.
电子在能层中的填充是从低能量到高能量进行的。根据这一规则,
n
+
ℓ
n + \ell
n+ℓ 值较低的亚能层比
n
+
ℓ
n + \ell
n+ℓ 值较高的亚能层先被填充。当
n
+
ℓ
n + \ell
n+ℓ 值相等时,
n
n
n 值较低的亚能层先被填充。
Q4: How many electrons does chlorine have?
氯有多少个电子?
There are 17 electrons present in a chlorine atom.
一个氯原子中有 17 个电子。
Q5: What is a valence shell with an example?
什么是价能层?请举例说明。
The outermost orbital shell of an atom is called its valence shell. These electrons take part in bonding with other atoms. Example: Sodium (Na), the electronic configuration of sodium is 1s² 2s² 2p⁶ 3s¹. The valence shell of Na is the M (3rd) shell.
原子的最外层轨道能层称为价能层。这些电子参与与其他原子的成键。例如钠(Na),钠的电子构型是 1s² 2s² 2p⁶ 3s¹。钠的价能层是 M(第三)能层。
内容总结
-
Electron Shell Basics: Electron shells are concentric energy levels around the nucleus, labeled as K, L, M, N, etc., corresponding to principal quantum numbers n = 1 , 2 , 3 , 4 n=1, 2, 3, 4 n=1,2,3,4, etc. Each shell has a maximum electron capacity determined by the formula 2n².
电子能层基础:电子能层是围绕原子核的同心能级,标记为 K、L、M、N 等,分别对应主量子数 n = 1 、 2 、 3 、 4 n=1、2、3、4 n=1、2、3、4 等。每个能层的最大电子容量由公式 2n² 确定。 -
Subshells and Orbitals: Shells are divided into subshells (s, p, d, f) defined by azimuthal quantum numbers ( ℓ \ell ℓ). Each subshell has a specific electron capacity: s (2), p (6), d (10), f (14), calculated by 2 ( 2 ℓ + 1 ) 2(2\ell + 1) 2(2ℓ+1) .
亚能层和轨道:能层分为由角量子数( ℓ \ell ℓ)定义的亚能层(s、p、d、f)。每个亚能层有特定的电子容量:s(2 个)、p(6 个)、d(10 个)、f(14 个),由公式 2 ( 2 ℓ + 1 ) 2(2\ell + 1) 2(2ℓ+1) 计算得出。 -
Electron Filling Rules: Electrons fill subshells following the Aufbau principle (lowest energy first, guided by n + l rule), Hund’s Rule, and Pauli Exclusion Principle.
电子填充规则:电子遵循泡利原理(按能量从低到高填充,由 n + l n + l n+l 规则指导)、洪德规则和泡利不相容原理填充亚能层。 -
Relation to Periodic Table: The periodic table reflects electron configurations. Periods correspond to new shell filling, and groups share similar valence electron configurations, leading to similar chemical properties.
与周期表的关系:周期表反映电子构型。周期对应新能层的填充,族内元素具有相似的价电子构型,因此化学性质相似。 -
Importance: Electron shell arrangements determine atomic size, ionization energy, electronegativity, and chemical bonding, with valence electrons playing a key role in chemical reactions.
重要性:电子能层排布决定原子大小、电离能、电负性和化学键合,价电子在化学反应中起关键作用。
What are Electron Configurations?
什么是电子排布?
What are Electron Configurations?
什么是电子排布?
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is
1s
2
2s
2
2p
6
3s
1
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^1
1s22s22p63s1.
元素的电子排布描述了电子在其原子轨道中的分布方式。原子的电子排布遵循一种标准表示法,在该表示法中,所有包含电子的原子亚层(其容纳的电子数标注在右上角作为上标)按顺序排列。例如,钠的电子排布式为
1s
2
2s
2
2p
6
3s
1
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^1
1s22s22p63s1。
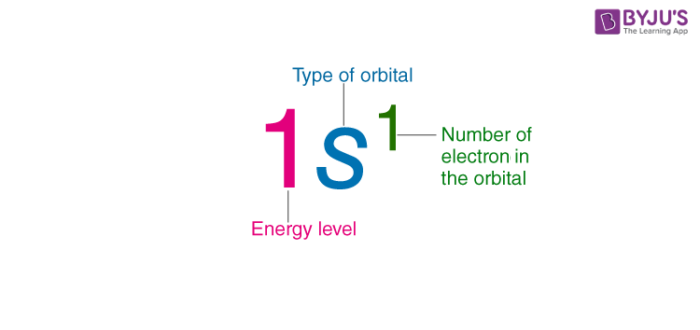
Type of orbital
轨道类型
Number of electron in the orbital
轨道中的电子数
Energy level
能级
1s 1 \text{1s}^1 1s1(1s 轨道上有 1 个电子)
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). In such cases, an abbreviated or condensed notation may be used instead of the standard notation. In the abbreviated notation, the sequence of completely filled subshells that correspond to the electronic configuration of a noble gas is replaced with the symbol of that noble gas in square brackets. Therefore, the abbreviated electron configuration of sodium is
[Ne]3s
1
\text{[Ne]3s}^1
[Ne]3s1 (the electron configuration of neon is
1s
2
2s
2
2p
6
\text{1s}^2 \text{2s}^2 \text{2p}^6
1s22s22p6, which can be abbreviated to
[He]2s
2
2p
6
\text{[He]2s}^2 \text{2p}^6
[He]2s22p6).
然而,标准表示法通常会使电子排布式变得冗长(尤其是对于原子序数较大的元素)。在这种情况下,可以使用简化式(或缩写式)代替标准表示法。在简化式中,与某一稀有气体电子排布相对应的、完全充满电子的亚层序列,会被该稀有气体的元素符号加方括号
[ ]
\text{[ ]}
[ ] 替代。因此,钠的简化电子排布式为
[Ne]3s
1
\text{[Ne]3s}^1
[Ne]3s1(氖的电子排布式为
1s
2
2s
2
2p
6
\text{1s}^2 \text{2s}^2 \text{2p}^6
1s22s22p6,其简化式可表示为
[He]2s
2
2p
6
\text{[He]2s}^2 \text{2p}^6
[He]2s22p6)。

Neon 氖: 1s 2 2s 2 2p 6 \text{1s}^2\text{2s}^2\text{2p}^6 1s22s22p6
Aluminum 铝: 1s 2 2s 2 2p 6 3s 2 3p 1 \text{1s}^2\text{2s}^2\text{2p}^6\text{3s}^2\text{3p}^1 1s22s22p63s23p1 → 简化为 [Ne] 3s 2 3p 1 \text{[Ne] 3s}^2\text{3p}^1 [Ne] 3s23p1
Argon 氩: 1s 2 2s 2 2p 6 3s 2 3p 6 \text{1s}^2\text{2s}^2\text{2p}^6\text{3s}^2\text{3p}^6 1s22s22p63s23p6
Calcium钙: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 \text{1s}^2\text{2s}^2\text{2p}^6\text{3s}^2\text{3p}^6\text{4s}^2 1s22s22p63s23p64s2 → 简化为 [Ar] 4s 2 \text{[Ar] 4s}^2 [Ar] 4s2
Electron Configurations are useful for:
电子排布的用途包括:
- Determining the valency of an element.
确定元素的化合价。 - Predicting the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties).
预测一组元素的性质(电子排布相似的元素往往表现出相似的性质)。 - Interpreting atomic spectra.
解释原子光谱。
This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
这种用于表示电子在原子轨道中分布的表示法,是在欧内斯特·卢瑟福(Ernest Rutherford)和尼尔斯·玻尔(Niels Bohr)于 1913 年提出玻尔原子模型后不久开始应用的。
Writing Electron Configurations
电子排布式的书写方法
Shells
电子层
The maximum number of electrons that can be accommodated in a shell is based on the principal quantum number (
n
n
n). It is represented by the formula
2
n
2
2n^2
2n2, where ‘
n
n
n’ is the shell number. The shells, values of
n
n
n, and the total number of electrons that can be accommodated are tabulated below.
一个电子层最多能容纳的电子数由主量子数(
n
n
n)决定,计算公式为
2
n
2
2n^2
2n2,其中
n
n
n 为电子层序数。电子层、对应的
n
n
n 值以及最多可容纳的电子数如下表所示。
| Shell and ‘
n
n
n’ value 电子层及 n n n 值 | Maximum electrons present in the shell 电子层最多可容纳电子 |
|---|---|
| K shell, n n n=1 | 2 × 1 2 = 2 2 \times 1^2 = 2 2×12=2 |
| L shell, n n n=2 | 2 × 2 2 = 8 2 \times 2^2 = 8 2×22=8 |
| M shell, n n n=3 | 2 × 3 2 = 18 2 \times 3^2 = 18 2×32=18 |
| N shell, n n n=4 | 2 × 4 2 = 32 2 \times 4^2 = 32 2×42=32 |
Subshells
亚层
The subshells into which electrons are distributed are based on the azimuthal quantum number (denoted by ‘
l
l
l’).
电子所分布的亚层由角量子数(用
l
l
l 表示)决定。
This quantum number is dependent on the value of the principal quantum number,
n
n
n. Therefore, when
n
n
n has a value of 4, four different subshells are possible.
角量子数的取值依赖于主量子数
n
n
n。因此,当
n
n
n=4 时,可能存在4个不同的亚层。
When
n
n
n=4. The subshells correspond to
l
l
l=0,
l
l
l=1,
l
l
l=2, and
l
l
l=3 and are named the s, p, d, and f subshells, respectively.
当
n
n
n=4 时,亚层对应的角量子数分别为
l
l
l=0、
l
l
l=1、
l
l
l=2、
l
l
l=3,对应的亚层名称分别为 s 亚层、p 亚层、d 亚层和 f 亚层。
The maximum number of electrons that can be accommodated by a subshell is given by the formula
2
×
(
2
l
+
1
)
2 \times (2l + 1)
2×(2l+1).
一个亚层最多可容纳的电子数由公式
2
×
(
2
l
+
1
)
2 \times (2l + 1)
2×(2l+1) 计算得出。
Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons, respectively.
因此,s 亚层、p 亚层、d 亚层和 f 亚层最多分别可容纳 2、6、10 和 14 个电子。
All the possible subshells for values of
n
n
n up to 4 are tabulated below.
n
n
n 值不超过4时所有可能的亚层如下表所示。
| Principle Quantum Number Value 主量子数( n n n)值 | Value of Azimuthal Quantum Number 角量子数( l l l)值 | Resulting Subshell in the Electron Configuration 电子排布中对应的亚层 |
|---|---|---|
| n n n=1 | l l l=0 | 1s \text{1s} 1s |
| n n n=2 | l l l=0 | 2s \text{2s} 2s |
| l l l=1 | 2p \text{2p} 2p | |
| n n n=3 | l l l=0 | 3s \text{3s} 3s |
| l l l=1 | 3p \text{3p} 3p | |
| l l l=2 | 3d \text{3d} 3d | |
| n n n=4 | l l l=0 | 4s \text{4s} 4s |
| l l l=1 | 4p \text{4p} 4p | |
| l l l=2 | 4d \text{4d} 4d | |
| l l l=3 | 4f \text{4f} 4f |
Thus, it can be understood that the
1p
\text{1p}
1p,
2d
\text{2d}
2d, and
3f
\text{3f}
3f orbitals do not exist because the value of the azimuthal quantum number is always less than that of the principal quantum number.
由此可知,
1p
\text{1p}
1p、
2d
\text{2d}
2d 和
3f
\text{3f}
3f 轨道并不存在,因为角量子数的取值始终小于主量子数。
Notation
表示方法
The electron configuration of an atom is written with the help of subshell labels.
原子的电子排布式通过亚层标识来书写。
These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript.
亚层标识包含电子层序数(由主量子数决定)、亚层名称(由角量子数决定)以及标注在右上角(作为上标)的亚层中总电子数。
For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘
1s
2
\text{1s}^2
1s2’.
例如,若第一电子层的 s 亚层填充了2个电子,对应的表示方法为
1s
2
\text{1s}^2
1s2。
With the help of these subshell labels, the electron configuration of magnesium (atomic number 12) can be written as
1s
2
2s
2
2p
6
3s
2
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^2
1s22s22p63s2.
借助这些亚层标识,镁(原子序数为12)的电子排布式可表示为
1s
2
2s
2
2p
6
3s
2
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^2
1s22s22p63s2。
Filling of Atomic Orbitals
原子轨道的填充规则
Aufbau Principle
能量最低原理
This principle is named after the German word ‘Aufbeen’ which means ‘build up’.
该原理的名称来源于德语单词“Aufbeen”,意为“逐步构建”。
The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals.
能量最低原理规定,电子会先占据能量较低的轨道,再占据能量较高的轨道。
The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers.
轨道的能量由主量子数与角量子数的和计算得出。
According to this principle, electrons are filled in the following order:
1s
\text{1s}
1s,
2s
\text{2s}
2s,
2p
\text{2p}
2p,
3s
\text{3s}
3s,
3p
\text{3p}
3p,
4s
\text{4s}
4s,
3d
\text{3d}
3d,
4p
\text{4p}
4p,
5s
\text{5s}
5s,
4d
\text{4d}
4d,
5p
\text{5p}
5p,
6s
\text{6s}
6s,
4f
\text{4f}
4f,
5d
\text{5d}
5d,
6p
\text{6p}
6p,
7s
\text{7s}
7s,
5f
\text{5f}
5f,
6d
\text{6d}
6d,
7p
\text{7p}
7p…
根据该原理,电子按以下顺序填充轨道:
1s
\text{1s}
1s、
2s
\text{2s}
2s、
2p
\text{2p}
2p、
3s
\text{3s}
3s、
3p
\text{3p}
3p、
4s
\text{4s}
4s、
3d
\text{3d}
3d、
4p
\text{4p}
4p、
5s
\text{5s}
5s、
4d
\text{4d}
4d、
5p
\text{5p}
5p、
6s
\text{6s}
6s、
4f
\text{4f}
4f、
5d
\text{5d}
5d、
6p
\text{6p}
6p、
7s
\text{7s}
7s、
5f
\text{5f}
5f、
6d
\text{6d}
6d、
7p
\text{7p}
7p……
The order in which electrons are filled in atomic orbitals as per the Aufbau principle is illustrated below.
根据能量最低原理,电子在原子轨道中的填充顺序如下图所示。
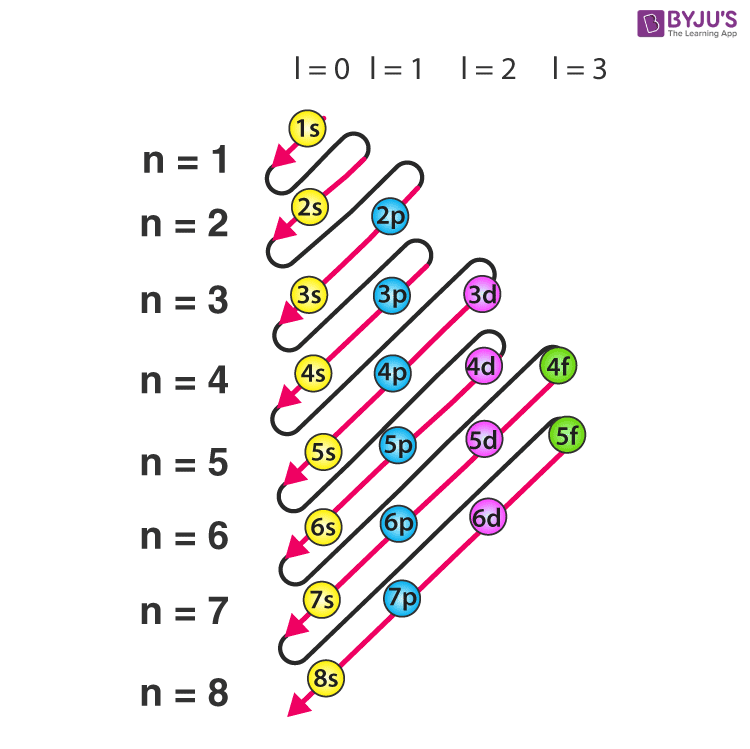
It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper. These exceptions can sometimes be explained by the stability provided by half-filled or completely filled subshells.
需要注意的是,能量最低原理存在许多例外情况,例如铬(Cr)和铜(Cu)。这些例外有时可通过半充满或全充满亚层所具有的稳定性来解释。
Pauli Exclusion Principle
泡利不相容原理
The Pauli exclusion principle states that a maximum of two electrons, each having opposite spins, can fit in an orbital.
泡利不相容原理指出,一个轨道最多只能容纳2个电子,且这2个电子的自旋方向必须相反。
This principle can also be stated as “no two electrons in the same atom have the same values for all four quantum numbers”.
该原理也可表述为“同一原子中,不存在4个量子数完全相同的两个电子”。
Therefore, if the principal, azimuthal, and magnetic numbers are the same for two electrons, they must have opposite spins.
因此,若两个电子的主量子数、角量子数和磁量子数均相同,则它们的自旋量子数必须相反。
Hund’s Rule
洪特规则
This rule describes the order in which electrons are filled in all the orbitals belonging to a subshell.
该规则描述了电子在同一亚层的所有轨道中的填充顺序。
It states that every orbital in a given subshell is singly occupied by electrons before a second electron is filled in an orbital.
洪特规则指出,在同一亚层中,所有轨道均先被单个电子占据后,才会有第二个电子填充到任意一个轨道中。
In order to maximize the total spin, the electrons in the orbitals that only contain one electron all have the same spin (or the same values of the spin quantum number).
为使总自旋角动量最大,仅填充单个电子的轨道中,所有电子的自旋方向均相同(即自旋量子数取值相同)。
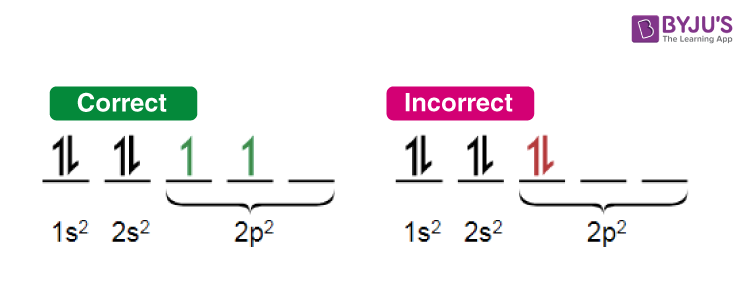
An illustration detailing the manner in which electrons are filled in compliance with Hund’s rule of maximum multiplicity is provided above.
上图详细展示了符合洪特最大多重度规则的电子填充方式。
- 轨道填充示意图
Correct 正确: 1s 2 2s 2 2p 2 \text{1s}^2 \text{2s}^2 \text{2p}^2 1s22s22p2(符合洪特规则的填充方式:2p 亚层的 3 个轨道各填充 1 个电子)
Incorrect 错误: 1s 2 2s 2 2p 2 \text{1s}^2 \text{2s}^2 \text{2p}^2 1s22s22p2(不符合洪特规则的填充方式:2p 亚层中 1 个轨道填 2 个电子,1 个轨道填 1 个电子,1 个轨道空着)
Representation of electronic Configuration of Atom
原子电子排布的表示方式
The electron configurations of a few elements are provided with illustrations in this subsection.
本部分将结合图示,给出几种元素的电子排布式。
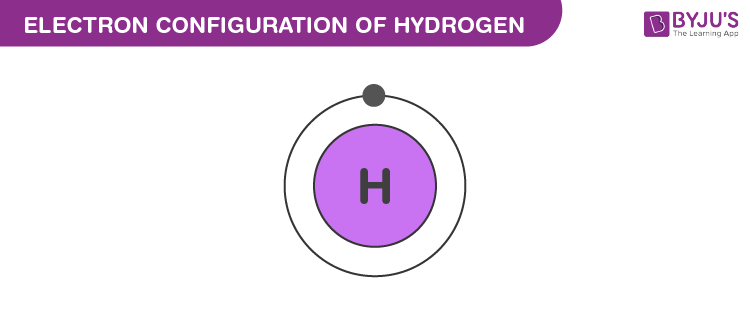
Electron Configuration of Hydrogen
氢的电子排布
The atomic number of hydrogen is 1. Therefore, a hydrogen atom contains 1 electron, which will be placed in the s subshell of the first shell/orbit. The electron configuration of hydrogen is
1s
1
\text{1s}^1
1s1, as illustrated below.
氢的原子序数为1,因此1个氢原子含有1个电子,该电子填充在第一电子层(或第一轨道)的s亚层中。氢的电子排布式为
1s
1
\text{1s}^1
1s1,如下图所示。
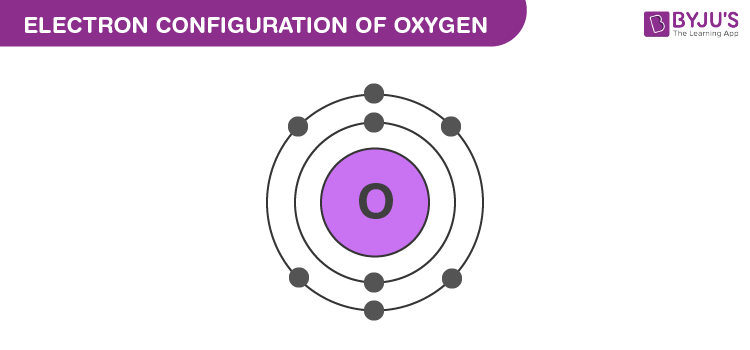
Electron Configuration of Oxygen
氧的电子排布
The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order:
氧的原子序数为8,意味着1个氧原子含有8个电子。其电子填充顺序如下:
- K shell – 2 electrons
K层(第一电子层)——2 个电子 - L shell – 6 electrons
L层(第二电子层)——6 个电子
Therefore, the electron configuration of oxygen is 1s 2 2s 2 2p 4 \text{1s}^2 \text{2s}^2 \text{2p}^4 1s22s22p4, as shown in the illustration provided below.
因此,氧的电子排布式为 1s 2 2s 2 2p 4 \text{1s}^2 \text{2s}^2 \text{2p}^4 1s22s22p4,如下图所示。
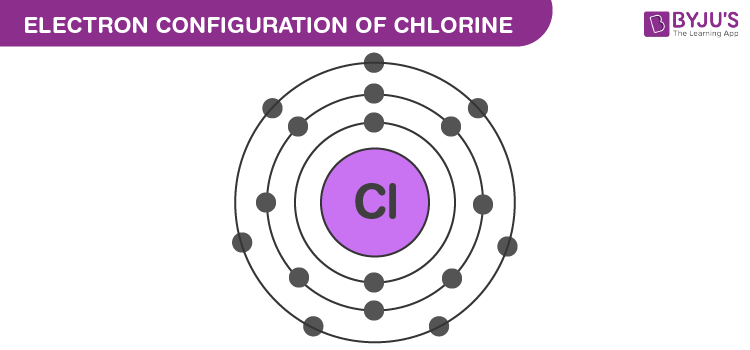
Chlorine Electronic Configuration
氯的电子排布
Chlorine has an atomic number of 17. Therefore, its 17 electrons are distributed in the following manner:
氯的原子序数为 17,因此其 17 个电子的分布方式如下:
- K shell – 2 electrons
K 层(第一电子层)——2 个电子 - L shell – 8 electrons
L 层(第二电子层)——8 个电子 - M shell – 7 electrons
M 层(第三电子层)——7 个电子
The electron configuration of chlorine is illustrated below. It can be written as
1s
2
2s
2
2p
6
3s
2
3p
5
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^2 \text{3p}^5
1s22s22p63s23p5 or as
[Ne]3s
2
3p
5
\text{[Ne]3s}^2 \text{3p}^5
[Ne]3s23p5.
氯的电子排布如下图所示,其电子排布式可表示为
1s
2
2s
2
2p
6
3s
2
3p
5
\text{1s}^2 \text{2s}^2 \text{2p}^6 \text{3s}^2 \text{3p}^5
1s22s22p63s23p5,或简化为
[Ne]3s
2
3p
5
\text{[Ne]3s}^2 \text{3p}^5
[Ne]3s23p5。
Thus, a brief introduction to electron configurations is provided in this article. To learn more about this topic and other related topics, such as Lewis dot structures, register with BYJU’S and download the mobile application on your smartphone.
至此,本文对电子排布进行了简要介绍。若想了解更多关于电子排布或其他相关主题(如路易斯结构式)的内容,可注册BYJU’S账号并在智能手机上下载其移动应用程序。
Frequently Asked Questions – FAQs
常见问题——FAQs
Q1
What is meant by the electronic configuration of an element?
元素的电子排布是什么意思?
The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals. While writing electron configurations, a standardized notation is followed in which the energy level and the type of orbital are written first, followed by the number of electrons present in the orbital written in superscript. For example, the electronic configuration of carbon (atomic number: 6) is
1s
2
2s
2
2p
2
\text{1s}^2 \text{2s}^2 \text{2p}^2
1s22s22p2.
元素的电子排布是一种符号表示法,用于表示该元素原子中的电子在不同原子轨道上的分布方式。书写电子排布式时,需遵循标准表示法:先写出能级和轨道类型,再在右上角标注该轨道中的电子数(作为上标)。例如,碳(原子序数为6)的电子排布式为
1s
2
2s
2
2p
2
\text{1s}^2 \text{2s}^2 \text{2p}^2
1s22s22p2。
Q2
What are the three rules that must be followed while writing the electronic configuration of elements?
书写元素电子排布式时必须遵循的三条规则是什么?
The three rules that dictate the manner in which electrons are filled in atomic orbitals are:
决定电子在原子轨道中填充方式的三条规则如下:
-
The Aufbau principle: electrons must completely fill the atomic orbitals of a given energy level before occupying an orbital associated with a higher energy level. Electrons occupy orbitals in the increasing order of orbital energy level.
能量最低原理:电子必须先完全填满某一能级的原子轨道,再占据更高能级的轨道。电子按轨道能量由低到高的顺序填充。 -
Pauli’s exclusion principle: states that no two electrons can have equal values for all four quantum numbers. Consequently, each subshell of an orbital can accommodate a maximum of 2 electrons and both these electrons MUST have opposite spins.
泡利不相容原理:同一原子中,不存在4个量子数完全相同的两个电子。因此,一个轨道的亚层最多可容纳2个电子,且这2个电子的自旋方向必须相反。 -
Hund’s rule of maximum multiplicity: All the subshells in an orbital must be singly occupied before any subshell is doubly occupied. Furthermore, the spin of all the electrons in the singly occupied subshells must be the same (in order to maximize the overall spin).
洪特最大多重度规则:同一亚层的所有轨道均先被单个电子占据后,才会有轨道被两个电子占据。此外,仅填充单个电子的轨道中,所有电子的自旋方向必须相同(以实现总自旋角动量最大)。
Q3
Why are electronic configurations important?
电子排布为什么重要?
Electron configurations provide insight into the chemical behaviour of elements by helping determine the valence electrons of an atom. It also helps classify elements into different blocks (such as the s-block elements, the p-block elements, the d-block elements, and the f-block elements). This makes it easier to collectively study the properties of the elements.
通过电子排布可以确定原子的价电子,从而帮助理解元素的化学行为;同时,电子排布还能帮助将元素划分为不同的区块(如s区元素、p区元素、d区元素和f区元素),这使得对元素性质的集中研究变得更加容易。
Q4
List the electron configurations of all the noble gases.
列出所有稀有气体的电子排布式。
The electronic configurations of the noble gases are listed below.
稀有气体的电子排布式如下:
- Helium (He) 氦 – 1s 2 \text{1s}^2 1s2
- Neon (Ne) 氖 – [He]2s 2 2p 6 \text{[He]2s}^2 \text{2p}^6 [He]2s22p6
- Argon (Ar) 氩 – [Ne]3s 2 3p 6 \text{[Ne]3s}^2 \text{3p}^6 [Ne]3s23p6
- Krypton (Kr) 氪 – [Ar]3d 10 4s 2 4p 6 \text{[Ar]3d}^{10} \text{4s}^2 \text{4p}^6 [Ar]3d104s24p6
- Xenon (Xe) 氙 – [Kr]4d 10 5s 2 5p 6 \text{[Kr]4d}^{10} \text{5s}^2 \text{5p}^6 [Kr]4d105s25p6
- Radon (Rn) 氡 – [Xe]4f 14 5d 10 6s 2 6p 6 \text{[Xe]4f}^{14} \text{5d}^{10} \text{6s}^2 \text{6p}^6 [Xe]4f145d106s26p6
Q5
What is the electronic configuration of copper?
铜的电子排布式是什么?
The electronic configuration of copper is
[Ar]3d
10
4s
1
\text{[Ar]3d}^{10} \text{4s}^1
[Ar]3d104s1. This configuration disobeys the aufbau principle due to the relatively small energy gap between the
3d
\text{3d}
3d and the
4s
\text{4s}
4s orbitals. The completely filled
d
\text{d}
d-orbital offers more stability than the partially filled configuration.
铜的电子排布式为
[Ar]3d
10
4s
1
\text{[Ar]3d}^{10} \text{4s}^1
[Ar]3d104s1。由于
3d
\text{3d}
3d 轨道和
4s
\text{4s}
4s 轨道之间的能量差较小,该排布式不符合能量最低原理。全充满的
d
\text{d}
d 轨道比部分填充的
d
\text{d}
d 轨道具有更高的稳定性。
核外电子排布规律
作者:垃圾本垃
一、初中阶段的核外电子排布规律
初中阶段学习的核外电子排布规律,其核心内容围绕核外电子排布图的绘制规则展开,具体如下:
- 电子优先填充内层轨道,再依次填充外层轨道。这是由于不同电子层的电子能量存在差异:外层电子能量较高,内层电子能量较低。为使原子体系处于能量最低的稳定状态,电子需优先占据能量更低的轨道。
- 各电子层容纳电子的数量存在上限:最内层(第一层)最多容纳 2 个电子,次外层最多容纳 8 个电子,最外层最多容纳 8 个电子。若最内层电子数为 3 个,或次外层、最外层电子数为 9 个,均不符合排布规则。
示例

前 18 号元素(短周期元素)的核外电子排布图
通过该图可总结出以下规律:元素所在的周期数等于原子的电子层数(仅限原子,不包含离子);主族序数等于原子最外层电子数(亦称价电子数);核电荷数、质子数、原子序数及核外电子数均相等。
(建议尝试绘制 S 离子的核外电子排布图以巩固知识。)
二、高中阶段的核外电子排布规律
随着科学理论的发展,高中阶段的核外电子排布规律更为复杂和精细。学习该部分内容需先掌握能层与能级的概念。
核外电子依据能量差异划分为不同能层,其表示符号为 K、L、M、N……能层由内向外依次排列,能层越高,电子能量越高,能量高低顺序为:
E ( K )< E ( L )< E ( M )< E ( N )< E ( O )< E ( P )< E ( Q ) E(K)<E(L)<E(M)<E(N)<E(O)<E(P)<E(Q) E(K)<E(L)<E(M)<E(N)<E(O)<E(P)<E(Q)
(对于高中阶段的学习要求,掌握前四个能层的符号顺序即可。)
同一能层的电子可进一步划分为不同能级,各能级的符号及所能容纳的最多电子数如下:

由图可知,任一能层的能级均从 s 能级开始,能级数与该能层序数相等;能级符号按 s、p、d、f……的顺序排列,符号前的数字表示其所属能层序数。各能级最多容纳的电子数为自然数中奇数数列 1、3、5、7……的 2 倍(即 2、6、10、14……),这是因为每个原子轨道最多可容纳 2 个电子,而 1、3、5、7……实际代表原子轨道的数量。
在多电子原子中,同一能层内各能级的能量顺序为:
E ( n s )< E ( n p )< E ( n d )< E ( n f ) E(ns)<E(np)<E(nd)<E(nf) E(ns)<E(np)<E(nd)<E(nf)
综上,核外电子的排布层次为:电子分布于原子轨道,轨道构成能级,能级组成能层,能层共同构成核外电子的整体分布。
注意
需明确区分 K、L、M、N 等能层符号与 s、p、d、f 等能级符号。
以下简要介绍原子轨道:原子轨道是电子填充的基本单位,每个轨道最多容纳 2 个电子,且这两个电子的自旋方向相反。自旋是微观粒子的固有属性,与电荷、质量类似。电子最终填充于轨道而非能级,能级的作用是规定轨道的形状和能量大小。
能级对轨道形状的规定如下:s 轨道呈球形,p 轨道呈哑铃状,具体如图所示:

s 电子的电子云轮廓图

三个相互垂直的 p 电子云
需注意,对于同一原子,能层越高,s 电子云的半径越大。这是由于电子能量升高后,在离核较远区域出现的概率相应增大。
能级对轨道能量的规定需结合构造原理说明:构造原理是以光谱学事实为基础,描述从氢原子开始,随着核电荷数递增,新增电子填入能级的顺序。

由于电子优先占据能量较低的轨道,根据构造原理可判断各轨道的能量高低。例如,4s 轨道的能量低于 3d 轨道,这种电子层数较大的轨道能量反而低于电子层数较小的轨道的现象,称为能级交错现象。
(需注意,当原子失去电子形成离子时,优先失去的是最外能层的电子,而非能量最高的电子。)
需要说明的是,构造原理所呈现的能级交错现象源于光谱学实验事实,属于经验总结,并非理论推导的结果,其本质是一种用于理解电子排布的思维模型和假想过程。
接下来引入能级组的概念:能级组是由能量相近的轨道组合而成,其构成形式为 n s n p ( n − 1 ) d ( n − 2 ) f ns \ np \ (n - 1)d \ (n - 2)f ns np (n−1)d (n−2)f。
能级组的具体应用将在后续章节中详细阐述。
(建议尝试书写短周期元素的电子排布式以加深理解。)
最后介绍原子实与简化电子排布式:稀有气体(如 He、Ne)的电子层结构为全充满的稳定结构。对于其他原子,可将其内层电子排布与某一稀有气体原子相同的部分称为原子实,并用该稀有气体的元素符号加方括号表示。在原子实基础上书写最外层电子排布,即可得到简化电子排布式。例如:
Na 的电子排布式为 1 s 2 2 s 2 2 p 6 3 s 1 1s^{2} \ 2s^{2} \ 2p^{6} \ 3s^{1} 1s2 2s2 2p6 3s1,因其内层电子排布与 Ne 相同,用原子实简化书写为 [ N e ] 3 s 1 [Ne] \ 3s^{1} [Ne] 3s1。
原子实之外的电子称为价电子。价电子属于原子中能量最高的能级组,且绝大多数价电子会参与化学反应中的电子得失,并决定元素的价态,因此研究价电子对确定元素性质具有重要意义。
编辑于 2022-08-19 09:54
泡利原理、洪特规则、能量最低原理
作者:垃圾本垃
泡利原理、洪特规则、能量最低原理这三大规则与前述构造原理的侧重点不同:三大规则主要解决电子在能级内的排布行为,而非电子填入哪个能级。例如,已知铁的电子排布式为 [ A r ] 3 d 6 4 s 2 [Ar]3d^{6}4s^{2} [Ar]3d64s2,那么 3 d 6 3d^{6} 3d6 中的 6 个电子如何填充?是填满 3 个轨道而剩余 2 个轨道空着,还是平均占据 5 个轨道后,剩余 1 个电子与其他电子成对?要解答这一问题,需先了解能量最低原理。
一、能量最低原理
前文已提及,基态是原子能量最低的状态。基态原子的电子排布遵循能量最低的原子轨道组合,据此可总结出能量最低原理:在构建基态原子时,电子将尽可能占据能量最低的原子轨道,使整个原子的能量处于最低状态。
例如,若一个电子越过 1 s 1s 1s 轨道直接填入 2 s 2s 2s 轨道,即违背了能量最低原理。该原理虽表述简单,却是物理学和化学领域的基础理论,也是后续两个规则的重要依据。
二、泡利不相容原理
1925 年,泡利(Pauli)提出:在一个原子轨道中,最多只能容纳 2 个电子,且这两个电子的自旋方向必须相反(关于自旋的概念详见前文),这一规律被称为泡利原理。
电子排布的轨道表达式(又称电子排布图)可进一步说明能级内电子的排布方式,其绘制规则如下:
- 用方框或圆圈表示原子轨道,能量相同的原子轨道(简并轨道)需用相连的方框表示;
- 用箭头表示电子的自旋状态,箭头方向代表自旋方向;
- 通常在方框的下方或上方标注能级符号,有时会通过能级的上下错落来体现能量高低差异。
示例如下:

在理解泡利原理和轨道表达式后,接下来学习洪特规则。
三、洪特规则
洪特(Hunt)指出:在基态原子或离子中,填入简并轨道的电子总是优先单独占据不同轨道,且自旋方向保持平行,这一规则称为洪特规则(例如,p 轨道为三重简并轨道,s 轨道为非简并轨道)。
需注意,洪特规则存在特例,且该特例是考试中的重点内容:当电子填充状态为半充满或全充满时,原子具有更高的稳定性。 例如,3 个电子填充 3 个轨道的状态比 4 个电子填充 3 个轨道的状态更稳定。
(建议尝试写出 21-36 号元素价电子的轨道表示式以巩固知识。)
在书写过程中会发现,Cu、Cr 两种元素的电子排布为特例,需特别注意其轨道表达式的正确书写。
必须强调,洪特规则仅适用于基态原子或离子,激发态不遵循该规则(但泡利不相容原理仍适用于激发态)。在激发态下,电子会重新排布并成对,此时原子的总能量并非各电子能量的简单叠加,体系的稳定状态取决于整体能量最低,而非单个电子的能量最低。
发布于 2022-08-19 23:51
via:
-
Why atomic orbits are named as k, l, m and n and so on?
https://medium.com/@nandakishore-r/why-atomic-orbits-are-named-as-k-l-m-and-n-and-so-on-3f873a1885a8 -
Why is the first shell called K shell?
https://www.vedantu.com/question-answer/first-shell-called-k-shell-class-11-chemistry-cbse-60d481f773e290701a853945 -
What are Shells?
https://byjus.com/chemistry/shell/ -
byjus.com/chemistry/electron-configuration/
https://byjus.com/chemistry/electron-configuration/ -
核外电子排布规律 - 知乎
https://zhuanlan.zhihu.com/p/554598064 -
泡利原理、洪特规则、能量最低原理 - 知乎
https://zhuanlan.zhihu.com/p/555513651





















 890
890

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








