Description:

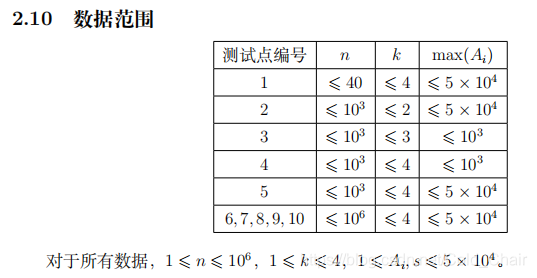
题解:
有gcd那先反演掉。
∑∑gcd(a,b)\sum\sum gcd(a, b)∑∑gcd(a,b)
=∑dd∗[d∣a]∗[d∣b]∗[(a/d,b/d)=1]=\sum_{d}d*[d|a]*[d|b]*[(a/d,b/d)=1]=∑dd∗[d∣a]∗[d∣b]∗[(a/d,b/d)=1]
=∑dd∗∑d′μ(d′)∗∑dd′∣a∑dd′∣bI=\sum_{d}d*\sum_{d'}\mu(d')*\sum_{dd'|a}\sum_{dd'|b}I=∑dd∗∑d′μ(d′)∗∑dd′∣a∑dd′∣bI
=∑d(∑d′∣dμ(d′)∗(d/d′))∑d∣a∑d∣b=\sum_{d}(\sum_{d'|d}\mu(d')*{(d/d')})\sum_{d|a}\sum_{d|b}=∑d(∑d′∣dμ(d′)∗(d/d′))∑d∣a∑d∣b
=∑dϕ(d)∗∑d∣a∗∑d∣b=\sum_{d}\phi(d)*\sum_{d|a}*\sum_{d|b}=∑dϕ(d)∗∑d∣a∗∑d∣b
其实这个也可以由∑d∣nϕ(d)=n\sum_{d|n}\phi(d)=n∑d∣nϕ(d)=n直接得来。
那么问题变成了枚举d,然后把d的倍数弄出来,使异或和为s。
我们只考虑k=4的情况,显然更小的k更简单。
先不考虑重复,之后再来减。
可以设阈值M,当d<=M的时候,d的倍数比较多,可以用FWT暴力统计,当d>M的时候,d的倍数比较少,可以meet-in-middle。
第一部分复杂度为O(M∗n∗log n)O(M*n*log~n)O(M∗n∗log n),
第二部分复杂度为∑i=M+1n(n/d)2\sum_{i=M+1}^{n}{(n/d)^2}∑i=M+1n(n/d)2
这个我不会化简,但是感受的到不会很大,随便平衡规划一下。
然后就是重复,注意s>0,所以只有两种情况:
1 3
1 1 2
那么只要枚举一个id,若s^(id)=j*d,算一下即可,系数也很好推。
Code:
#include<cstdio>
#include<algorithm>
#define pp printf
#define ll long long
#define fo(i, x, y) for(int i = x; i <= y; i ++)
#define ff(i, x, y) for(int i = x; i < y; i ++)
using namespace std;
const int mo = 998244353;
ll ksm(ll x, ll y) {
ll s = 1;
for(; y; y /= 2, x = x * x % mo)
if(y & 1) s = s * x % mo;
return s;
}
ll ni2;
const int N = 65536, M = 128;
int n, k, s, x;
int cnt[N];
ll a[N], b[N]; int tp, r[N];
void dft(ll *a, int tp, int F) {
int n = 1 << tp;
for(int h = 1; h < n; h *= 2)
for(int j = 0; j < n; j += 2 * h) {
ll A, *l = a + j, *r = a + j + h;
ff(i, 0, h) {
A = *r, *r = (*l - A + mo) % mo, *l = (*l + A) % mo;
if(F == -1) *l = *l * ni2 % mo, *r = *r * ni2 % mo;
l ++, r ++;
}
}
}
void fwt(ll *a, ll *b, int tp) {
int n = 1 << tp;
dft(a, tp, 1); dft(b, tp, 1);
ff(i, 0, n) a[i] = a[i] * b[i] % mo;
dft(a, tp, -1);
}
int bz[N], p[N], phi[N], m;
void sieve(int n) {
fo(i, 2, n) {
if(!bz[i]) p[++ p[0]] = i, phi[i] = i - 1;
for(int j = 1; i * p[j] <= n; j ++) {
int k = i * p[j]; bz[k] = 1;
if(i % p[j] == 0) {
phi[k] = phi[i] * p[j];
break;
}
phi[k] = phi[i] * phi[p[j]];
}
}
phi[1] = 1;
}
ll ans, sum;
ll c2(ll n) {
return n * (n - 1) / 2 % mo;
}
ll c3(ll n) {
return n * (n - 1) * (n - 2) / 6 % mo;
}
ll pe[N], ni24;
int gcd(int x, int y) {
return !y ? x : gcd(y, x % y);
}
ll g[N];
int c[1000005];
int main() {
freopen("choose.in", "r", stdin);
freopen("choose.out", "w", stdout);
ni2 = ksm(2, mo - 2);
ni24 = ksm(24, mo - 2);
scanf("%d %d %d", &n, &k, &s);
m = 65535; tp = 16;
fo(i, 1, n) scanf("%d", &x), cnt[x] ++, c[i] = x;
if(k == 1) {
pp("%lld\n", (ll) cnt[s] * s % mo);
return 0;
}
if(k == 2) {
fo(i, 0, m) ans += (ll) cnt[i] * cnt[s ^ i] % mo * gcd(i, s ^ i) % mo;
pp("%lld\n", ans % mo * ni2 % mo);
return 0;
}
if(k == 3) {
fo(i, 1, n) fo(j, i + 1, n) {
int p = s ^ c[i] ^ c[j];
if(p > 5e4) continue;
if(cnt[p]) ans += gcd(c[i], gcd(c[j], p)) * cnt[p];
if(p == c[i]) ans -= gcd(p, c[j]);
if(p == c[j]) ans -= gcd(p, c[i]);
}
pp("%lld\n", ans / 3);
return 0;
}
sieve(m);
fo(d, 1, m) {
sum = 0;
if(d <= M) {
ff(i, 0, 1 << tp) a[i] = b[i] = 0;
fo(i, 1, m / d) a[i * d] = b[i * d] = cnt[i * d];
fwt(a, b, tp);
fo(i, 0, m) sum = (sum + a[i] * a[s ^ i]) % mo;
} else {
fo(i, 1, m / d) fo(j, 1, m / d)
g[(i * d) ^ (j * d)] += (ll)cnt[i * d] * cnt[j * d] % mo;
fo(i, 1, m / d) fo(j, 1, m / d)
sum += g[s ^ (i * d) ^ (j * d)] % mo * cnt[i * d] % mo * cnt[j * d] % mo;
fo(i, 1, m / d) fo(j, 1, m / d)
g[(i * d) ^ (j * d)] -= (ll) cnt[i * d] * cnt[j * d] % mo;
}
int sp = 0; fo(i, 1, m / d) sp += cnt[d * i];
fo(i, 1, m / d) {
if((s ^ (i * d)) % d != 0) continue;
int j = (s ^ (i * d)) / d; if(i > j) continue;
if(!cnt[i * d] || !cnt[j * d]) continue;
sum -= (ll) cnt[i * d] * cnt[j * d] % mo * 8 % mo;
sum -= (ll) cnt[i * d] * cnt[j * d] % mo * (sp - 2) % mo * 12 % mo;
}
sum = (sum % mo + mo) % mo;
ans = (ans + sum * phi[d]) % mo;
}
pp("%lld", ans * ni24 % mo);
}





 本文深入解析了在数学算法竞赛中如何利用反演的思想解决涉及最大公约数(GCD)的问题,通过详细步骤展示了从公式推导到具体实现的过程,包括使用FWT算法进行快速统计和meet-in-middle技巧处理复杂情况,最后提供了完整的C++代码实现。
本文深入解析了在数学算法竞赛中如何利用反演的思想解决涉及最大公约数(GCD)的问题,通过详细步骤展示了从公式推导到具体实现的过程,包括使用FWT算法进行快速统计和meet-in-middle技巧处理复杂情况,最后提供了完整的C++代码实现。
















 612
612

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








