Alzheimer’s disease (AD) is a protein misfolding pathology, caused by accumulation of abnormally folded Aβ and tau polypeptides, which form amyloid plaques and neurofibrillary tangles in the brain of affected individuals. Aβ aggregates have been linked with learning and memory deficits in both human and mouse models of the disease, making Aβ deposition a target for prevention and treatment [1–3]. In the last years, a lot of effort has been focused on the identification of the processes leading to Aβ aggregation. Evidence have indicated that, although Aβ42 is a highly aggregating form of Aβ [4, 5], the co-occurrence of Aβ peptides with different length can affect the neurotoxic and aggregation potential of the Aβ pool (reviewed in [6]). As an example, changes in the ratio of Aβ40/42 has been shown to represent an important factor in initializing Aβ fibrillogenesis and toxicity [7], indicating that the presence of different Aβ forms may affect the development of AD in vivo.
Consistently, while small amounts of Aβ-containing brain extracts, deriving from either AD patient or AD transgenic mouse, induce β-amyloidosis and glial activation once intracranially injected in pre-depositing AD transgenic mice [8–11], the chronic infusion of soluble, synthetic Aβ42 peptides into wild type (wt) rodent brains does not result in amyloid deposition [9]. The finding that Aβ42 alone fails to show seeding properties in the healthy brain and does not trigger pathogenetic pathways indicates the occurrence of efficient clearance mechanisms and suggests that brain-specific cofactors, specifically present in pathological conditions, are needed for effective seeding [9].
阿尔茨海默病(AD)是一种蛋白质错误折叠病理,由异常折叠的aβ和tau多肽的积累引起,这些多肽在受影响的个体的大脑中形成淀粉样蛋白斑块和神经原纤维缠结。在人类和小鼠疾病模型中,Aβ聚集体与学习和记忆缺陷有关,使Aβ沉积成为预防和治疗的目标[1-3]。在过去的几年里,许多工作都集中在识别导致aβ聚集的过程上。有证据表明,尽管Aβ42是Aβ的高度聚集形式[4,5],但不同长度的Aβ肽的共存会影响Aβ库的神经毒性和聚集潜力(综述见[6])。例如,Aβ40/42比值的变化已被证明是启动Aβ原纤维形成和毒性的一个重要因素[7],表明不同Aβ形式的存在可能影响体内AD的发展。
一致地,尽管来自AD患者或AD转基因小鼠的少量含Aβ的脑提取物在预沉积的AD转基因小鼠脑内注射后会诱导β-淀粉样变性和神经胶质活化[8-11],但将可溶性合成Aβ42肽长期输注到野生型(wt)啮齿动物脑中不会导致淀粉样蛋白沉积[9]。单独的Aβ42不能在健康大脑中显示出播种特性,也不会触发致病途径,这一发现表明了有效清除机制的发生,并表明大脑特异性辅因子,特别是在病理条件下存在,是有效播种所必需的[9]。
Beta-Amyloid 1-42, scrambled 是 Beta-Amyloid 1-42 的无活性乱序序列,与 Beta-Amyloid 1-42 活性片段具有相同的氨基酸组成,常用作阴性对照。
名称:Scramble-Beta-Amyloid 1-42
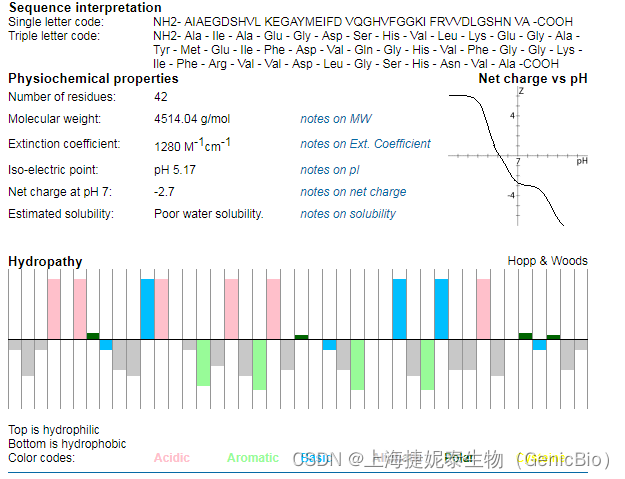
序列(单字母):AIAEGDSHVLKEGAYMEIFDVQGHVFGGKIFRVVDLGSHNVA
序列(三字母): H-Ala-Ile-Ala-Glu-Gly-Asp-Ser-His-Val-Leu-Lys-Glu-Gly-Ala-Tyr-Met-Glu-Ile-Phe-Asp-Val-Gln-Gly-His-Val-Phe-Gly-Gly-Lys-Ile-Phe-Arg-Val-Val-Asp-Leu-Gly-Ser-His-Asn-Val-Ala-OH
CAS: 1678415-52-5
分子式:C203H311N55O60S
分子量:4514.14
Scrambled-Beta-Amyloid (1-42)序列分析:
引用文献:
1, Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–9.
2, Scheuner D, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8):864–70.
3, Selkoe DJ. Aging, amyloid, and Alzheimer’s disease: a perspective in honor of Carl Cotman. Neurochem Res. 2003;28(11):1705–13.
4, Bitan G, et al. Amyloid beta-protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003;100(1):330–5.
5, Yan Y, Wang C. Abeta42 is more rigid than Abeta40 at the C terminus: implications for Abeta aggregation and toxicity. J Mol Biol. 2006;364(5):853–62.
6, Hubin E, et al. Transient dynamics of Abeta contribute to toxicity in Alzheimer’s disease. Cell Mol Life Sci. 2014;71(18):3507–21.
7, Kuperstein I, et al. Neurotoxicity of Alzheimer’s disease Abeta peptides is induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J. 2010;29(19):3408–20.
8, Kane MD, et al. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. J Neurosci. 2000;20(10):3606–11.
9, Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313(5794):1781–4.
10, Eisele YS, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106(31):12926–31.
11, Watts JC, et al. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2011;108(6):2528–33.

产品链接:http://www.genicbio.cn/detail.html?article_id=88
上海捷妮泰生物科技有限公司(GenicBio Limited)
微 信: 199 1653 7972
QQ: 1704635669
邮 箱: service@genicbio.cn
网 址: www.genicbio.cn




 文章探讨了阿尔茨海默病中的Aβ蛋白质错误折叠路径,特别是Aβ42聚合体与学习记忆缺陷的关系。研究指出,不同长度的Aβ肽比例对神经毒性有影响,且在健康大脑中,Aβ42本身不具备引发病理进程的能力,暗示病理状态下特定的脑内因子对于Aβ聚集至关重要。
文章探讨了阿尔茨海默病中的Aβ蛋白质错误折叠路径,特别是Aβ42聚合体与学习记忆缺陷的关系。研究指出,不同长度的Aβ肽比例对神经毒性有影响,且在健康大脑中,Aβ42本身不具备引发病理进程的能力,暗示病理状态下特定的脑内因子对于Aβ聚集至关重要。

















 3万+
3万+

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








