
知识点:
1.原子结构构造,质子、中子、电子相关概念
2.符号xyA的识别及各符号的意义
3.认识原子轨道形状,掌握电子排布式、轨道排布式写法
4.掌握电离能、电负性的概念
题型一:符号xyA的应用(主要以选择题为主)

答案:B
题型二:电子排布式(简答为主)
An atom,X,has a proton number of 16.Deduce the electron configuration of this atom.
答案:1s 22s 22p 63s23p4
题型三:电离能、电负性(选择简答为主)
The graph below shows the variation of the first ionisation energy with the number of protons for some elements.
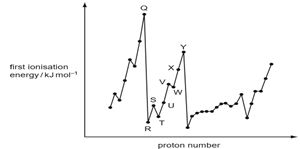
Which statement is corred?
A. Elements Q and Y are in the same period in the Periodic Table.
B. The general increase from elements R to Y is due to increasing atomic radius.
C. The small decrease between dldments S and T is due to decreased shielding.
D. The small decrease between elements V and W is due to repulsion between paired electrons.
答案:D





 本文探讨了原子结构中的基础知识,包括质子、中子、电子的构成,以及xyA符号的含义。深入解析电子排布式和轨道排布,涉及选择题中的符号应用、电子配置的推导,以及电离能和电负性的概念和实例分析。通过实例帮助读者掌握相关知识点和解题技巧。
本文探讨了原子结构中的基础知识,包括质子、中子、电子的构成,以及xyA符号的含义。深入解析电子排布式和轨道排布,涉及选择题中的符号应用、电子配置的推导,以及电离能和电负性的概念和实例分析。通过实例帮助读者掌握相关知识点和解题技巧。
















 277
277

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








